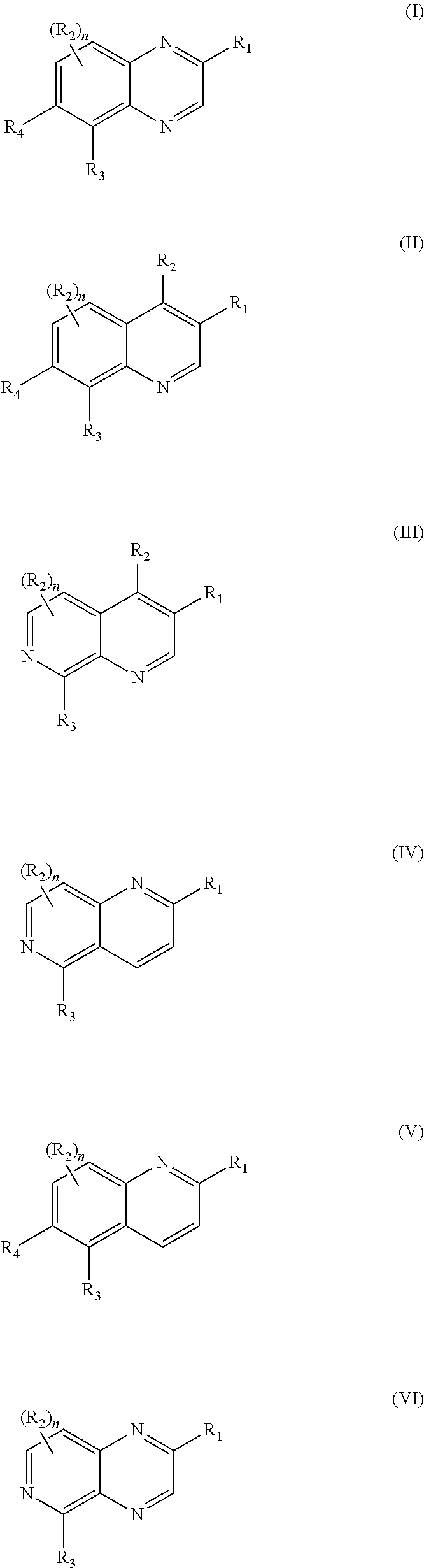
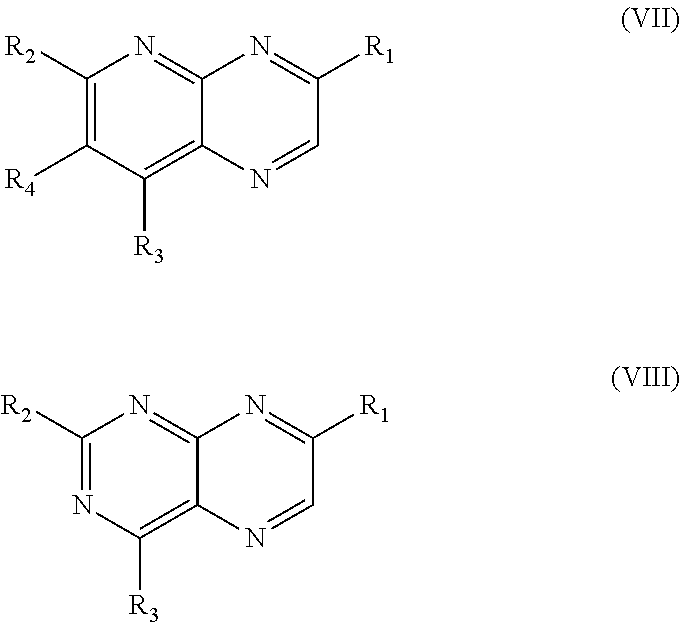
Tricyclic heteroaryl-substituted quinoline and azaquinoline compounds as par4 inhibitors
a technology of azaquinoline and quinoline, which is applied in the field of tricyclic heteroaryl substituted compounds, can solve the problems of limited efficacy, increased risk of bleeding, and limited current anti-platelet therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0830]Intermediate 1B (70 mg, 0.121 mmol) was subject to a chiral SFC separation using the following condition: Instrument: Berger Multigram II Prep SFC Column: Chiralpak AS-H, 30×250 mm, 5 micron; Mobile Phase: 30% MeOH / 70% CO2; Flow Conditions: 85 mL / min, 150 Bar, 40° C.; Detector Wavelength: 234 nm. Two peaks were obtained corresponding to the two enantiomers. The fast eluting fraction (RT=18.5 min) was combined, concentrated, lyophilized to give Example 1 (26 mg, 0.043 mmol, 35.3% yield) as a slightly yellow solid. 1H NMR (400 MHz, THF) δ 8.80 (br. s., 1H), 8.67 (d, J=1.5 Hz, 1H), 8.47 (s, 1H), 8.06 (br. s., 1H), 7.73 (d, J=7.3 Hz, 1H), 7.68 (d, J=0.9 Hz, 1H), 7.05 (s, 1H), 6.55 (d, J=8.8 Hz, 1H), 4.51-4.44 (m, 2H), 4.35 (d, J=4.6 Hz, 2H), 4.18 (dd, J=11.9, 7.7 Hz, 1H), 4.00 (s, 3H), 3.72 (s, 3H), 2.55 (s, 3H); LC-MS: BEH C18 2.1×50 mm; A: water+0.05% TFA; B: acetonitrile+0.05% TFA; wavelength 220 nm; flow rate 0.8 mL / min; gradient time 1.5 min; 2 to 98% B. RT=1.27 min, MS (ESI)...
example 2
(S)-(4-chloro-2-(2-methoxy-7-methylquinoxalin-5-yl)-7,8-dihydro-[1,4]dioxino[2′,3′:3,4]benzo[1,2-d]thiazol-7-yl)methyl (6-methoxypyridin-3-yl)carbamate
[0831]
[0832]Example 2 was obtained from the second (slow eluting fraction, RT=22.7 min) peak in the separation of Intermediate 1B (27.6 mg, 0.045 mmol, 37.5% yield): 1H NMR (400 MHz, THF) δ 8.91 (br. s., 1H), 8.78 (d, J=2.0 Hz, 1H), 8.58 (s, 1H), 8.16 (br. s., 1H), 7.84 (d, J=8.4 Hz, 1H), 7.80-7.76 (m, 1H), 7.15 (s, 1H), 6.66 (d, J=8.8 Hz, 1H), 4.62-4.54 (m, 2H), 4.45 (d, J=4.8 Hz, 2H), 4.29 (dd, J=11.8, 7.8 Hz, 1H), 4.11 (s, 3H), 3.83 (s, 3H), 2.66 (s, 3H); LC-MS: BEH C18 2.1×50 mm; A: water+0.05% TFA; B: acetonitrile+0.05% TFA; wavelength 220 nm; flow rate 0.8 mL / min; gradient time 1.5 min; 2 to 98% B. RT=1.27 min, MS (ESI) m / z: 580.1 (M+H)+. Analytical HPLC (method A): RT=12.36 min, 95% purity.
Example 3
(2-(2-methoxy-7-methylquinoxalin-5-yl)-4-methyl-7,8-dihydro-[1,4] dioxino [2′,3′: 3,4]benzo [1,2-d]thiazol-7-yl)methyl (2-hydroxypy...
example 3
[0836]To a solution of 4-aminopyridin-2-ol (18.67 mg, 0.170 mmol) in DCM (0.8 mL) was added DIEA (0.074 mL, 0.424 mmol), followed by addition of Intermediate 3A (20 mg, 0.042 mmol) in THF (0.8 mL). The reaction mixture was stirred at room temperature for 1.0 h. HPLC and LCMS indicated a completion of reaction. The reaction was quenched by addition of a small amount of MeOH / water / 0.1% TFA. Solvent was removed under vacuum. The crude was dissolved in DMSO and purified via preparative LC / MS (method D, 70-100% B over 10 minutes, then a 5-minute hold at 100% B). Fractions containing the desired product were combined and dried via centrifugal evaporation to yield Example 3 (12.0 mg). 1H NMR (500 MHz, DMSO-d6) δ 10.63 (br. s., 1H), 8.75 (s, 1H), 8.59 (s, 1H), 8.24 (d, J=5.5 Hz, 1H), 7.83 (s, 1H), 7.58 (s, 1H), 7.43 (d, J=5.8 Hz, 1H), 6.99 (s, 1H), 4.67 (br. s., 1H), 4.61 (d, J=11.6 Hz, 1H), 4.56-4.43 (m, 2H), 4.33-4.26 (m, 1H), 4.09 (s, 3H), 2.69 (s, 3H), 2.65 (s, 3H); LC-MS: method H, RT=...
PUM
| Property | Measurement | Unit |
|---|---|---|
| aromaticity | aaaaa | aaaaa |
| Rh | aaaaa | aaaaa |
| Ri | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap