Methods and compositions for enhancing functional myelin production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Nuclease Mediated PLP1 Inactivation to Treat Genetic Myelin Disorders
Introduction
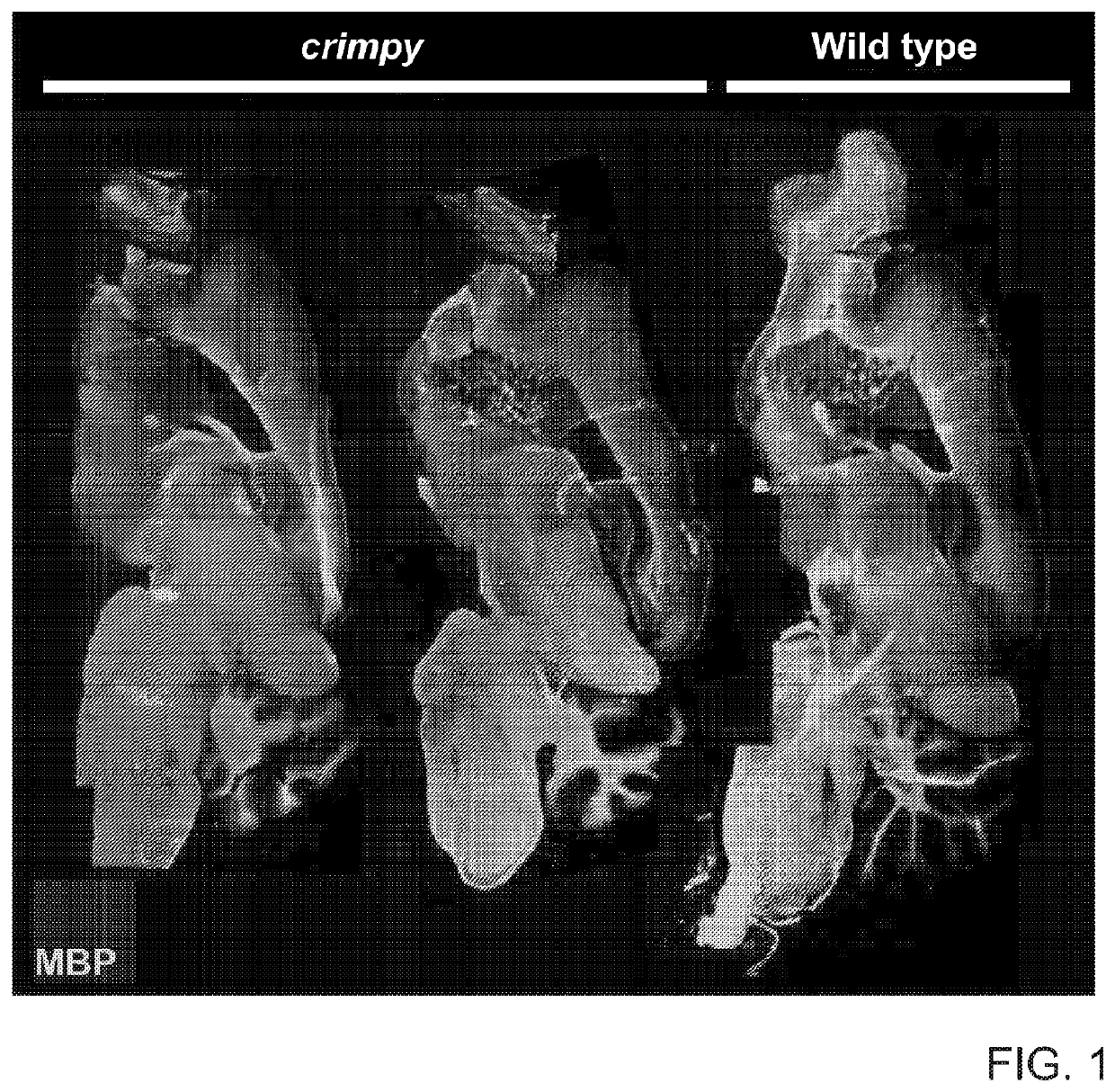
[0328]Abnormal myelination results in aberrant neuronal-signaling and neurological dysfunction. Restoration of the myelinating-capacity in these patients by correction of the genetic abnormalities within their endogenous, myelinating cells represents a promising curative avenue, however the feasibility of this therapeutic approach has yet to be demonstrated. Therefore, we chose to validate this paradigm by focusing on a severe, archetypal leukodystrophy called Pelizaeus-Merzbacher Disease (PMD).
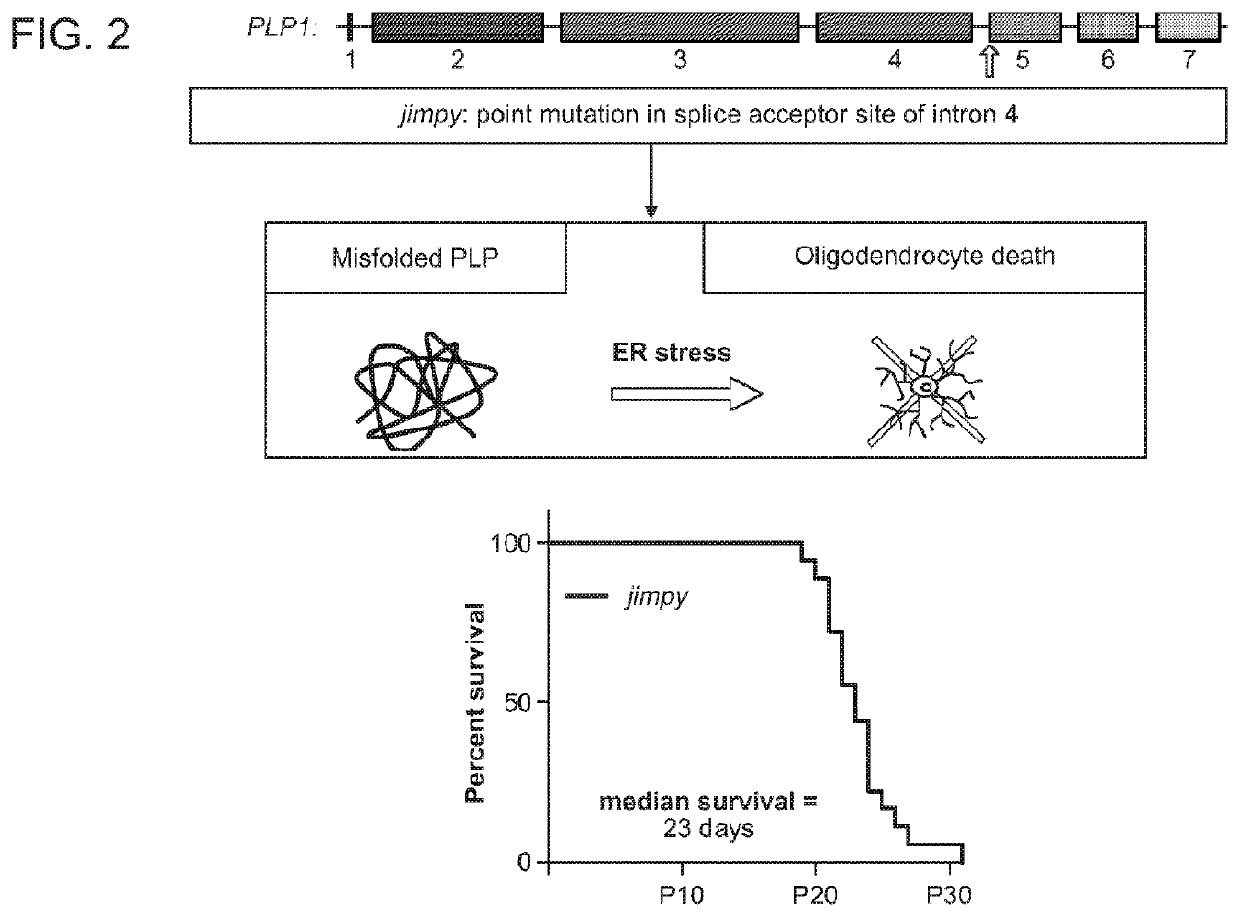
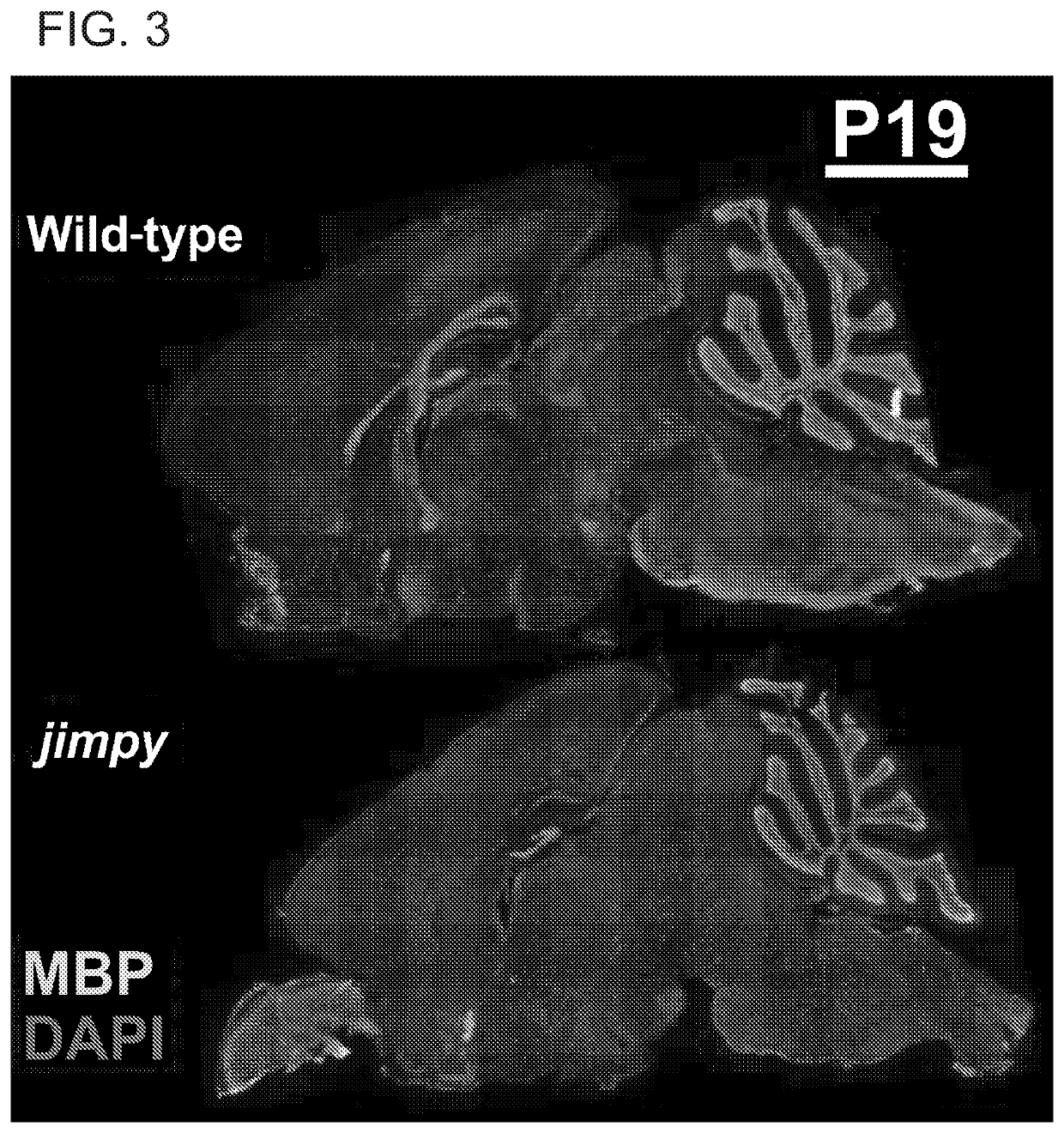
[0329]PMD is a severe X-linked genetic disorder of myelin caused by mutations in the proteolipid protein 1 (PLP1). PMD patients typically experience severe neurological disease, including profound cognitive and motor disability, which invariably culminates in early mortality during childhood or adolescence. Therefore, we looked to determine if severe PMD patients could be treated through introduction of nuclease ...
example 2
Post-Natal Inactivation of PLP1 Using AAV Delivery of CRISPR / Cas9
[0352]This example demonstrates that post-natal inactivation of PLP1 can be used to effectively treat PMD, or reduce the severity of PMD.
[0353]To facilitate the delivery of the CRISPR / Cas9 system with sgRNA targeting the PLP1 locus in a patient, several CNS-targeted AAV serotypes, including PHP.B, were generated, and AAV tropism for OPCs were validated. The AAV constructs (AAV9 or AAV-PHP.B) contain a SaCRISPR-Cas9 nuclease (CMV-SaCas9, SaCas9 coding sequence under the control of a CMV promotor) with a site directed guide RNA (sgRNA) against PLP1 (U6-sgRNA, the sgRNA is under the control of the U6 promotor), which is designed to generate indels in the PLP1 gene and thus prevent expression of the defective PLP1 protein.
[0354]Mice were maintained in accordance with approved protocols reviewed by Case Western Reserve University's Institutional Animal Care and Use Committee. Mice were housed in a temperature and humidity c...
example 3
Post-Natal Knockdown of PLP1 Using Antisense Oligonucleotides (ASO)
[0362]This example demonstrates that post-natal down-regulation or knockdown of PLP1 gene activity using ASO can be used to effectively treat PMD, or reduce the severity of PMD.
[0363]Mice are maintained in accordance with approved protocols reviewed by Case Western Reserve University's Institutional Animal Care and Use Committee. Mice are housed in a temperature and humidity controlled housing unit under a 12 hour day / light cycle and are allowed ad libitum access to food.
[0364]Male postnatal day 0 pups are obtained from jimpy (a severe mouse model of Pelizaeus Merzbacher Disease) breeding pairs and rapidly anesthetized using cryoanesthesia. A 10 μL Hamilton syringe with a 32 gauge needle is loaded with antisense oligonucleotides targeting PLP1. The needle is lowered through the skull to a depth of about 2 mm at a position 2 / 5 from the intersection of the saggital suture and lambdoid to the eye. 2 μL of ASO solution i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com