A multiplexed diagnostic assay for iron and vitamin a deficiency and methods of use thereof
a diagnostic assay and multiplex technology, applied in the field of multiplexed diagnostic assay for iron and vitamin a deficiency, can solve the problems of difficult to obtain the test for each of the markers at the same time, difficult to measure these targets on the same test device, etc., and achieve the effect of rapid and accurate quantification of iron, vitamin a and inflammation status, and convenient operation of tools
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ed Micronutrient Deficiency Test Strip Architecture and Testing Procedure
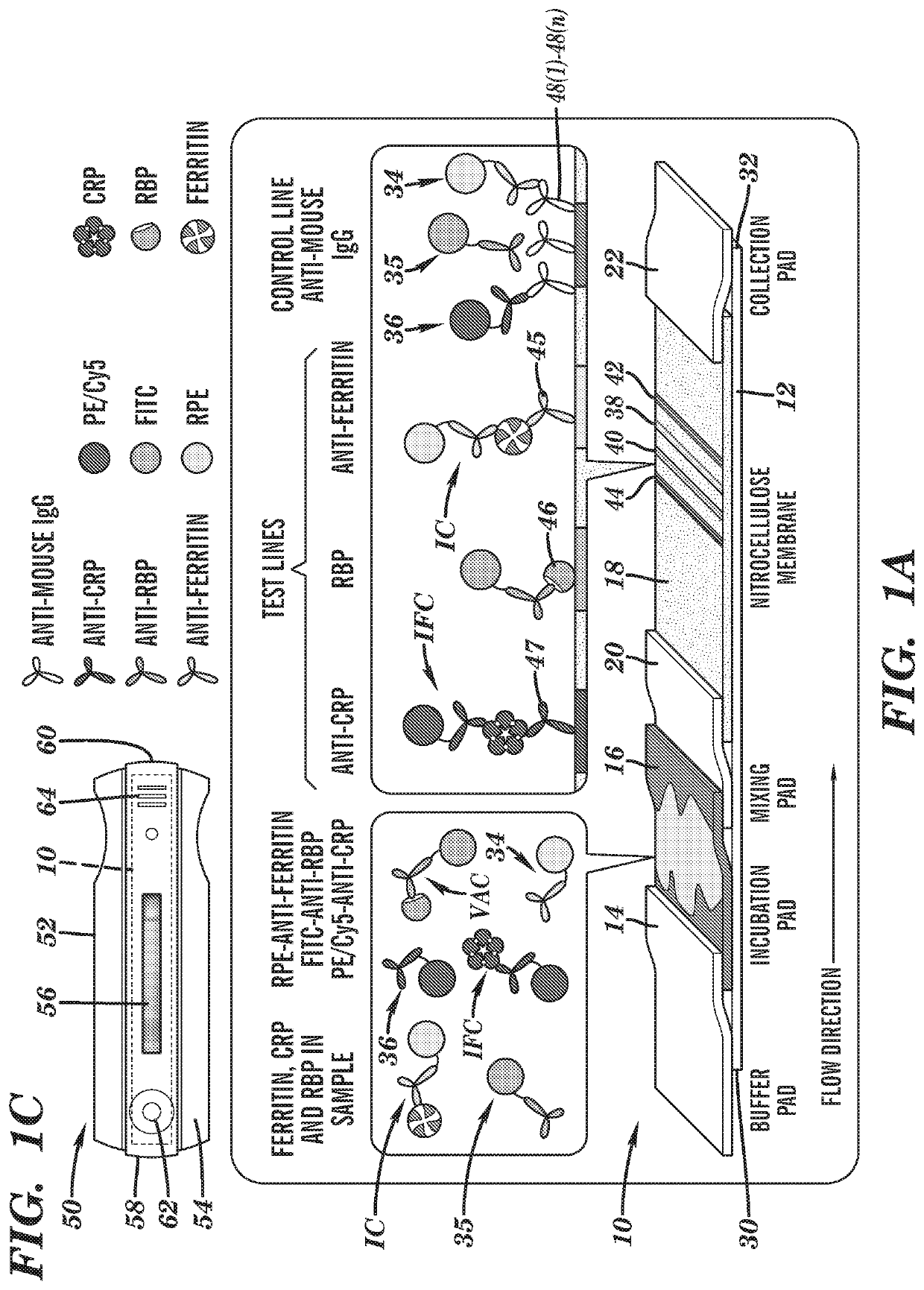
[0065]Tests were performed using a disposable custom multiplexed fluorescence test strip that measures ferritin, RBP and CRP concentrations. The test strip consists of: a buffer pad that accepts running buffer, an incubation pad that incubates sample with labeled antibodies, a mixing pad, a nitrocellulose membrane with immobilized anti-CRP, RBP protein, anti-ferritin and secondary antibodies sequentially in the direction of flow, and another cellulose fiber pad to collect the waste sample at the end. To simplify operation an incubation pad is incorporated in the test strip as a substitute for the sample and conjugation pads traditionally used in lateral flow test strips. The incubation pad is pre-loaded with labeled antibodies, allowing pre-incubation of the sample and labeled antibodies immediately as soon as the sample was added.
[0066]To perform the test, first 15 μL of human serum was added on the incubation...
example 2
d Fluorescence Imaging System
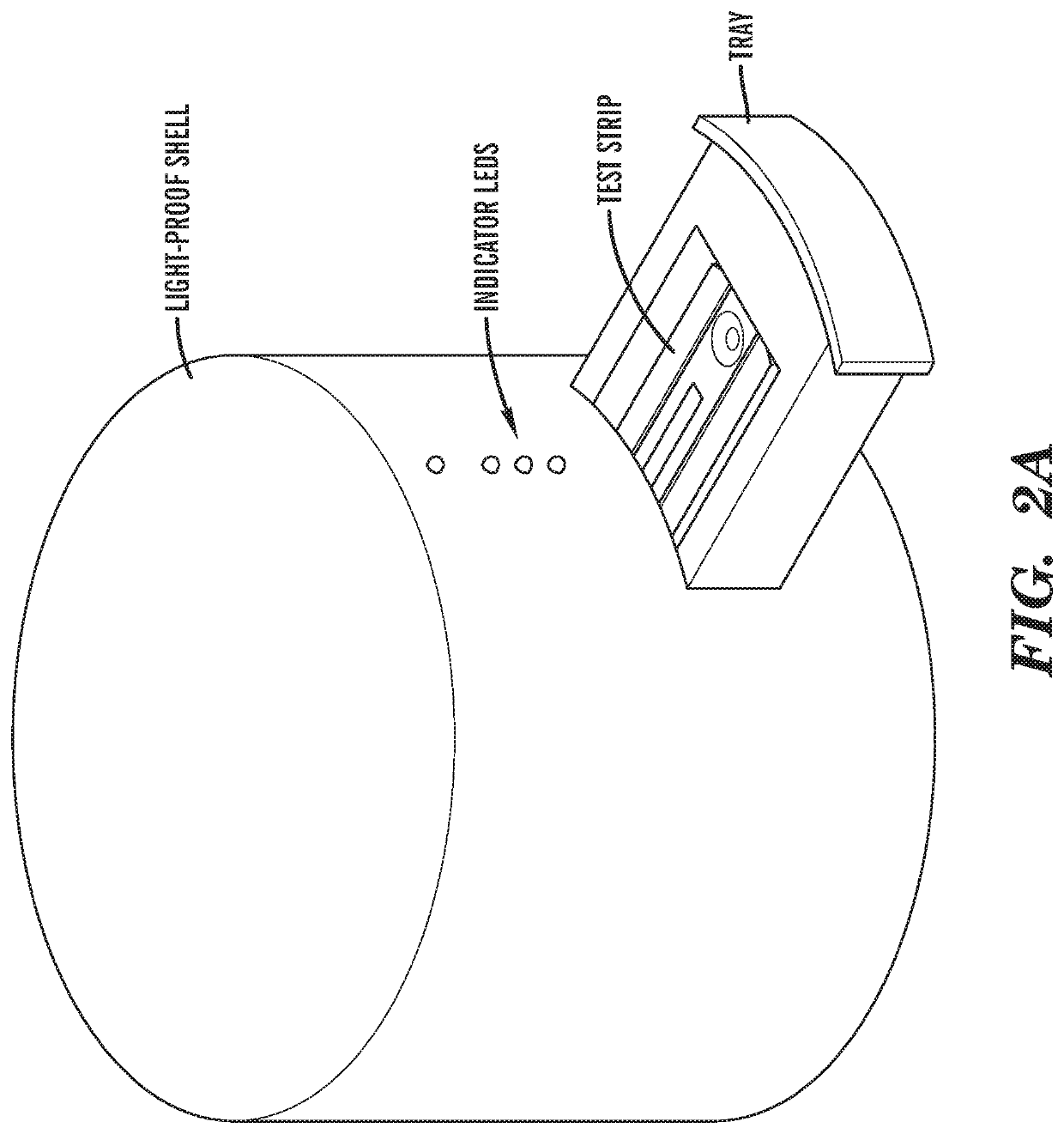
[0067]FIGS. 2A and 2B show the design of an exemplary reader device that was used to analyze the assay. The reader links up with a standard laptop or the technology disclosed in U.S. Patent Application Publication No. 2016 / 0080548 and PCT Patent Application PCT / US14 / 12263, the disclosures of which are hereby incorporated by reference in their entirety herein, to interpret the results and display them to the user.
[0068]In the reader, a tray is built to accept test strip cartridges with a variety of shapes. As shown in FIG. 2C, fluorescence signals appear on the test strip only during fluorescence imaging mode. FIG. 2D shows the design of the fluorescence detection system. The sensor was developed using a Raspberry Pi camera module and is controlled by software using the PiCamera open source library. The sensor excites the fluorescence signal on the test strip using six blue LEDs covered by band pass optical filters with a center wavelength at 458 nm. The ...
example 3
RBP and CRP Assay Quantification
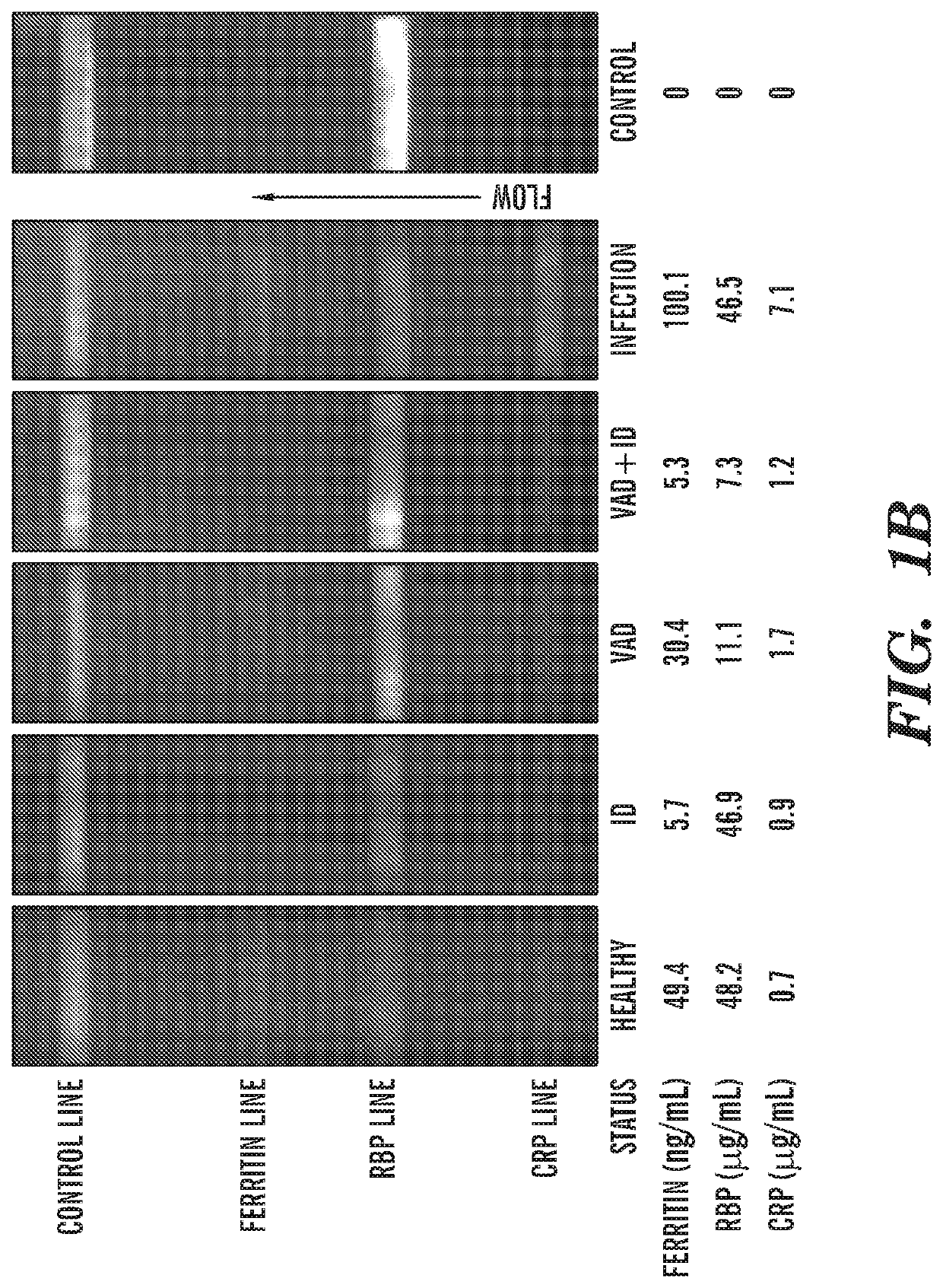
[0071]43 human whole blood samples from different participants were used to quantify the assay. The blood samples were purchased from a commercial source (Research Blood Components, LLC), and were all from US adult donors with no appearance of infectious disease. Concentrations of ferritin, CRP and RBP in the samples were characterized with commercial ELISA kits (Abcam, Inc.). No data was excluded. Four batches of test strips were manufactured and randomly selected for each test. The test strips were stored in light-free environment at room temperature until used. No significant batch to batch variability between test strips was observed, and storage up to 6 weeks had no noticeable effect on the test result. Human serum samples were separated with a portable centrifuge from whole blood and then used as direct input in to the test. FIG. 3A shows the colorimetric variation of test lines from three different human serum samples with known ferritin, RBP a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com