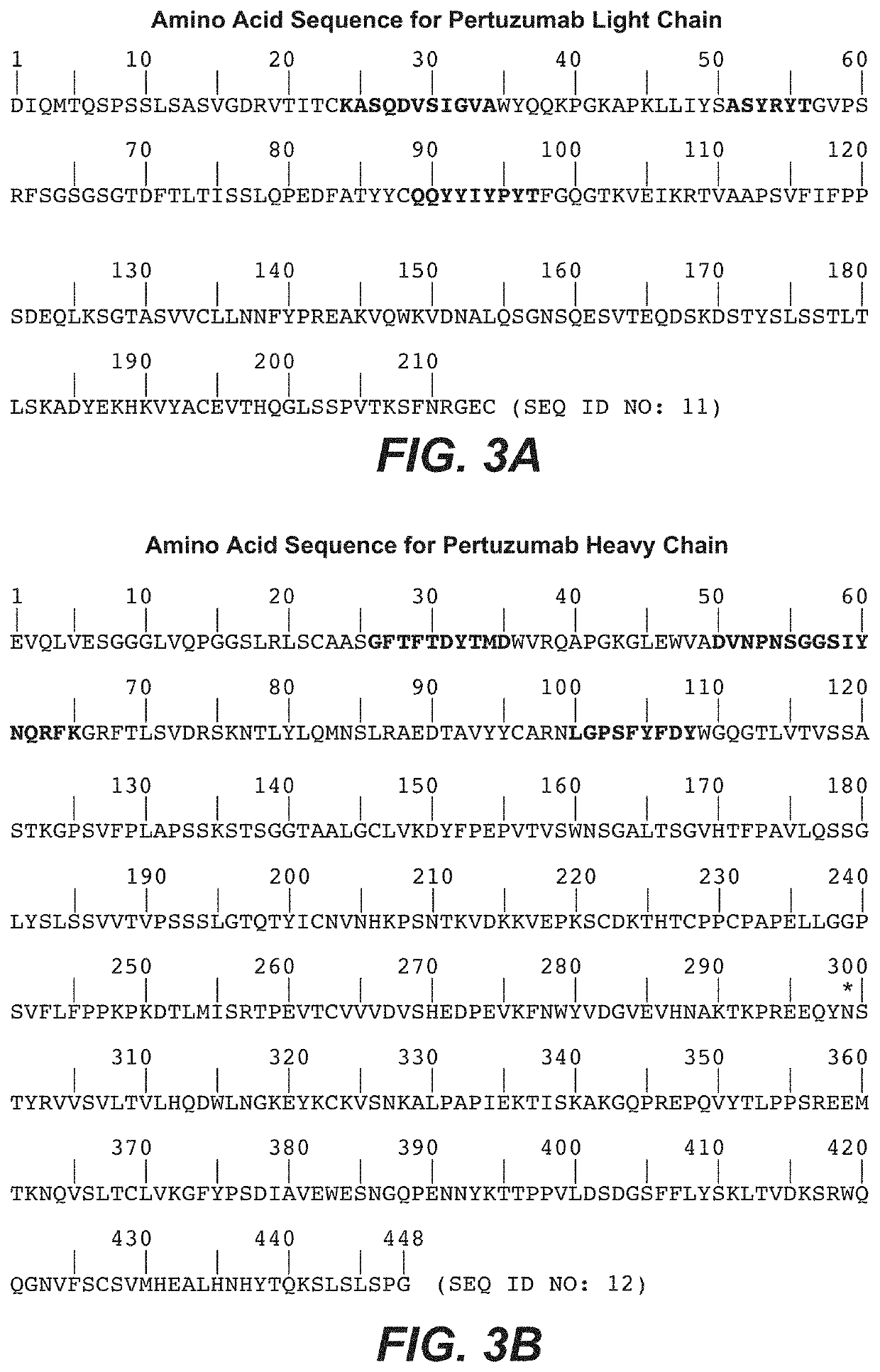
Subcutaneous her2 antibody formulations
a technology of her2 antibody and subcutaneous injection, which is applied in the direction of immunoglobulins against animals/humans, drug compositions, peptides, etc., can solve the problems of reducing tumor proliferation and survival, and achieve the effect of increasing the dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Phase I Pertuzumab Subcutaneous Dose-Finding Study in Combination with Trastuzumab
[0311]This is a Phase I, open-label, two-part multicenter clinical pertuzumab subcutaneous dose-finding study in combination with trastuzumab in healthy male volunteers and female patients with early breast cancer.
[0312]The study is designed the safety and PK of pertuzumab SC for Q3W treatment by applying a PK-based approach to compare an SC formulation to the approved W formulation. In this dose-finding study, it is intended to identify the SC dose that is comparable to IV with respect to serum concentrations. pertuzumab Q3W SC serum Ctrough concentrations are unknown.
[0313]Different types of pertuzumab SC injections will be assessed in the study:[0314]Separate administration of pertuzumab SC with or without trastuzumab SC as separate injections (co-administration)[0315]Simultaneous administration of pertuzumab SC and trastuzumab SC as single injection (co-mixed)[0316]Administration of pertuzumab and ...
example 2
Stable Subcutaneous Fixed-Dose Co-Formulations (SC FDC) of Pertuzumab and Trastuzumab
[0730]Stable fixed-dose co-formulations (FDC) of pertuzumab and trastuzumab were developed for subcutaneous (SC) administration.
[0731]The co-formulation studies used the pertuzumab and trastuzumab SC Drug Substance (DS) compositions and rHuPH20 composition shown in FIG. 19.
[0732]The amount (%) of high molecular weight species (HMWS) in various subcutaneous pertuzumab and trastuzumab formulations, and pertuzumab / trastuzumab co-formulations containing trehalose and / or sucrose as stabilizer at 5° C. and 25° C. are shown in FIG. 23.
[0733]The following SC FDC loading and maintenance formulations were found to be stable and suitable for subcutaneous administration of a single co-formulation of pertuzumab and trastuzumab to human patients:
[0734]Loading Dose
[0735]Pertuzumab[0736]Dose: 1,200 mg[0737]Concentration: 80 mg / mL
[0738]Trastuzumab[0739]Dose: 600 mg[0740]Concentration: 40 mg / mL
[0741]rHuPH20[0742]Conc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap