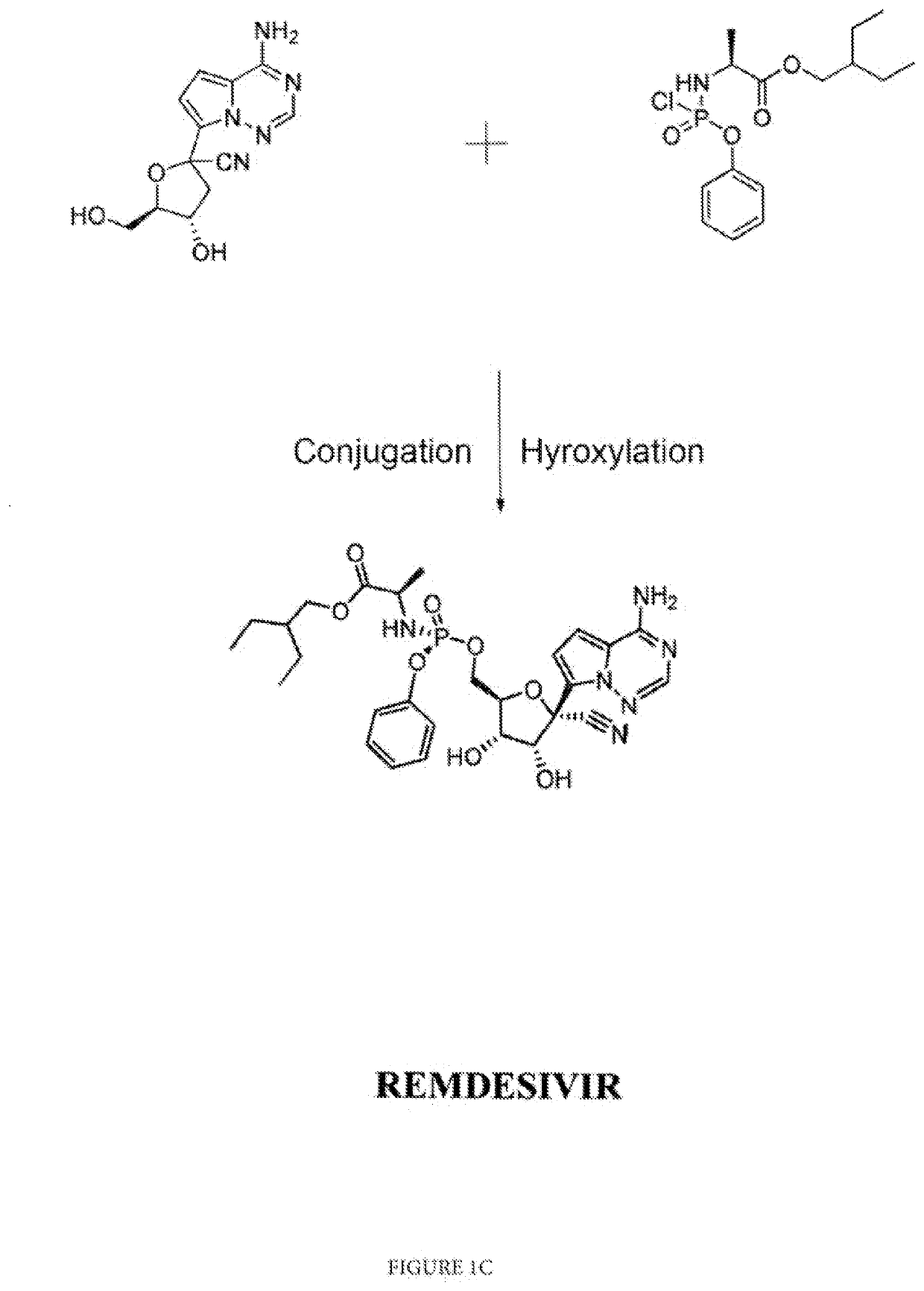
Isomorphs of remdesivir and methods for synthesis of same
a technology of isomorphs and remdesivir, which is applied in the field of new isomorphs of 2ethylbutyl (), can solve the problems of viral infections caused by, arenaviridae, coronaviridae, filoviridae, etc., and achieve the effect of improving the aqueous solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
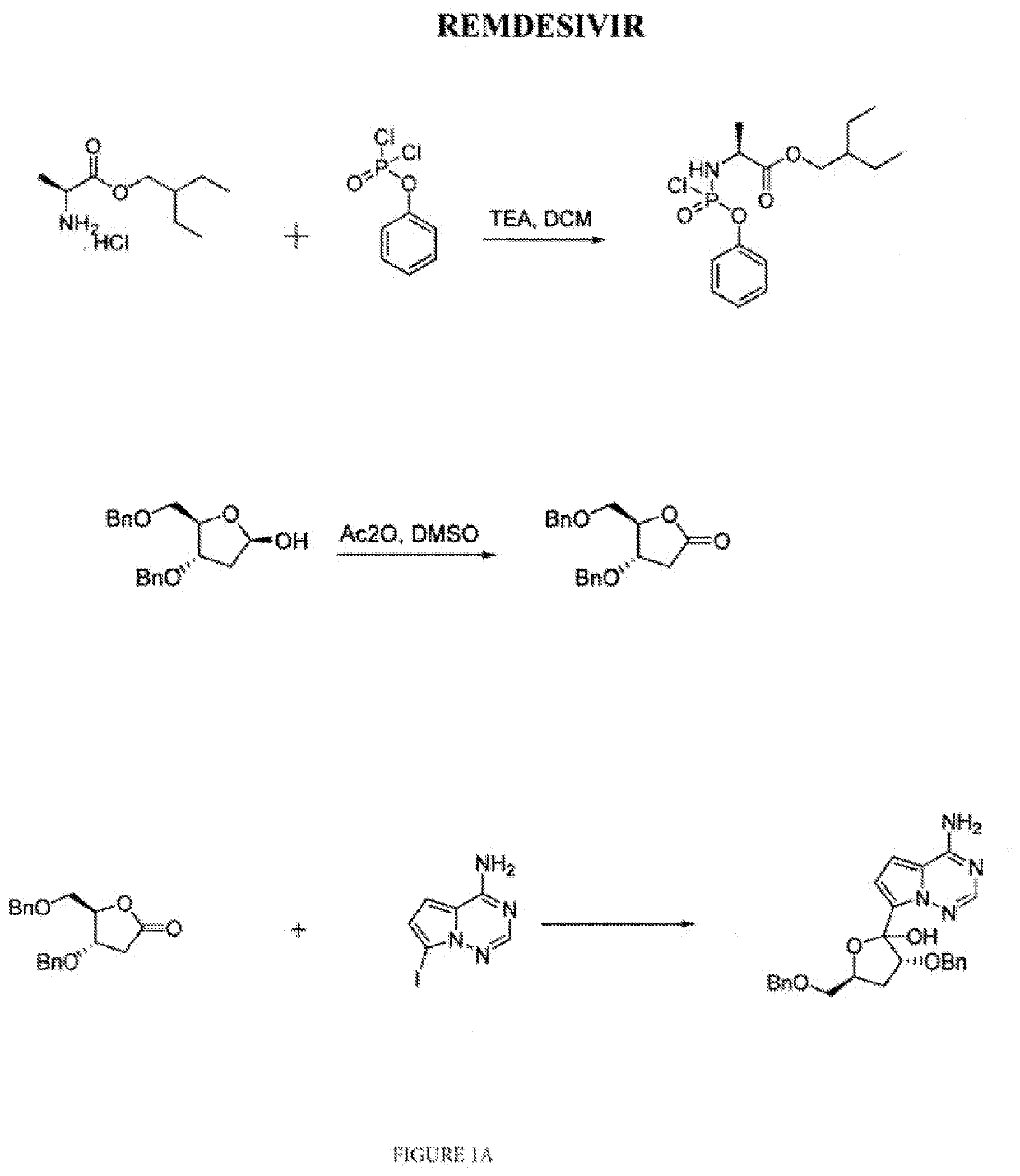
on of 2-ethylbutyl (chloro(phenoxy)phosphoryl)-L-alaninate
[0082]2-ethylbutyl (chloro(phenoxy)phosphoryl)-L-alaninate was prepared by the process reported in US′360. Accordingly, Phenyl dichlorophosphate was 20 reacted with 2-ethylbutyl alanine ester in presence of trimethylamine and methylene dichloride solvent to obtain 2-ethylbutyl (chloro(phenoxy)phosphoryl)-L-alaninate.
example 2
on of (3R,4R,5R)-3,4-bis(benzyloxy)-5-((benzyloxy) methyl) dihydrofuran-2(3H)-one
[0083](3R,4R,5R)-3,4-bis(benzyloxy)-5-((benzyloxy)methyl)dihydrofuran-2(3H)-one was prepared by the process reported in US′360. Accordingly, (3R,4R,5R)-3,4-bis (benzyloxy)-5-((benzyloxy)methyl) tetrahydrofuran 2-ol was reacted with acetic anhydride in DMSO to obtain (3R,4R,5R)-3,4-bis(benzyloxy)-5-((benzyloxy)methyl)dihydrofuran-2(3H)-one.
example 3
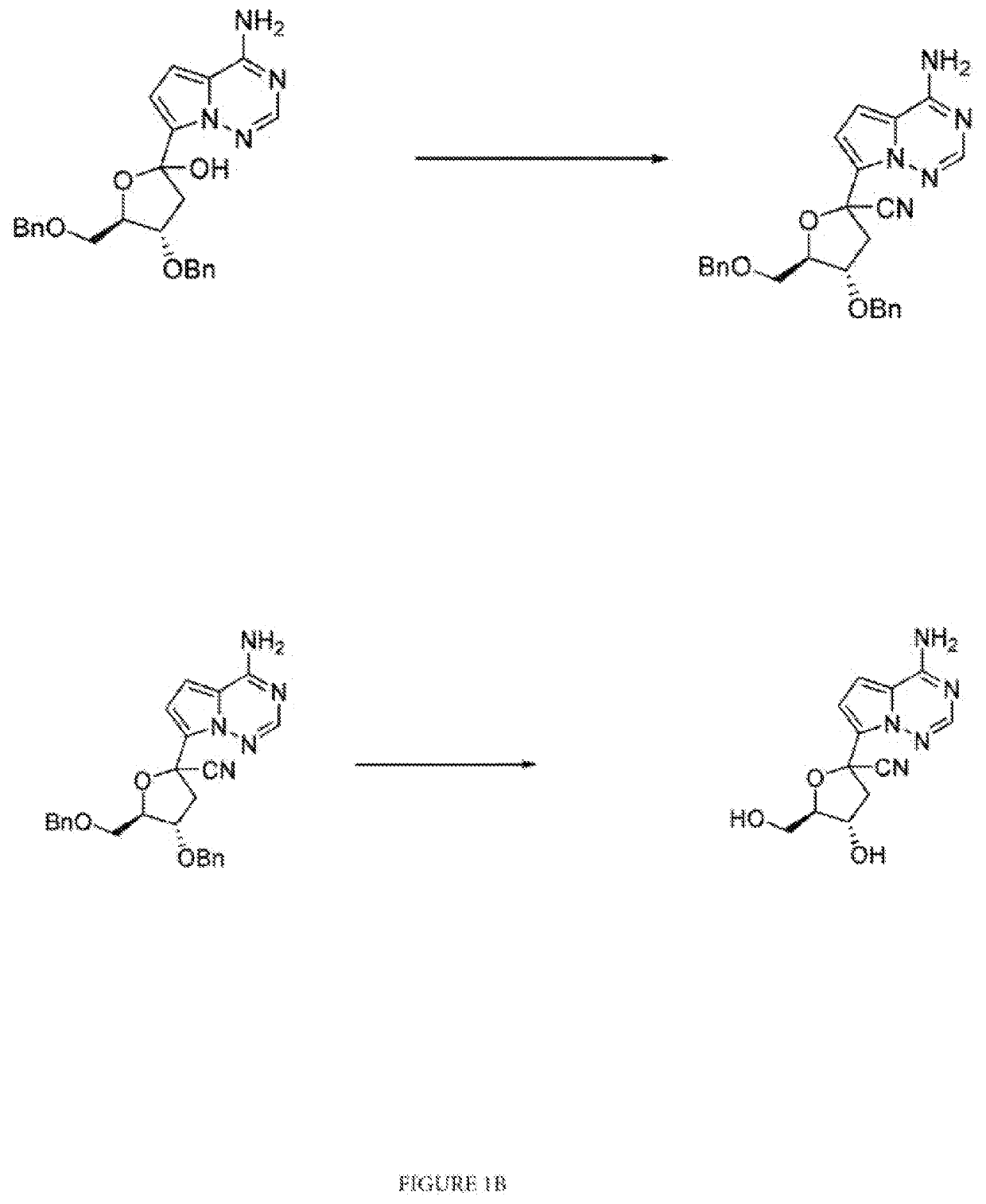
on of (3R,4R,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-bis(benzyloxy)-5-((benzyloxy)methyl)tetrahydrofuran-2-carbonitrile
[0084]To the compound of example 2 (50 gm) was charged MDC and the reaction mass was cooled to −75±5° C. followed by addition of trifluoromethane sulfonic acid (15 ml) at same temperature, stirred to get the thick mass. To the mass was added TMSOTf (12.5 ml), stirred at same temperature, added TMSCN (50 ml), stirred reaction mass for 2 hrs. After completion of reaction, was added TEA (40 ml) at −75±5° C., raised the temperature to 30±5° C., charged NaHCO3 (50 gm), water (300 ml) and allowed to settle the mass at room temperature. The reaction mass was extracted with MDC, washed the organic layer with 10% NaCl solution, distilled the organic layer under vacuum below 45° C., cooled, dried to obtain the product. Yield: 60-65 gm; HPLC purity: NLT 98.0%
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap