Treating stroke and other diseases without inhibiting N-type calcium channels
a technology of n-type calcium channel and stroke, which is applied in the direction of metabolism disorder, peptide/protein ingredient, peptide source, etc., can solve the problems of clinical trials with these drugs in stroke and traumatic brain injury that have so far failed, and no therapeutic treatment has been approved. to achieve the effect of facilitating uptake of an active agen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Screening for Side Effects of Tat-NR2B9c
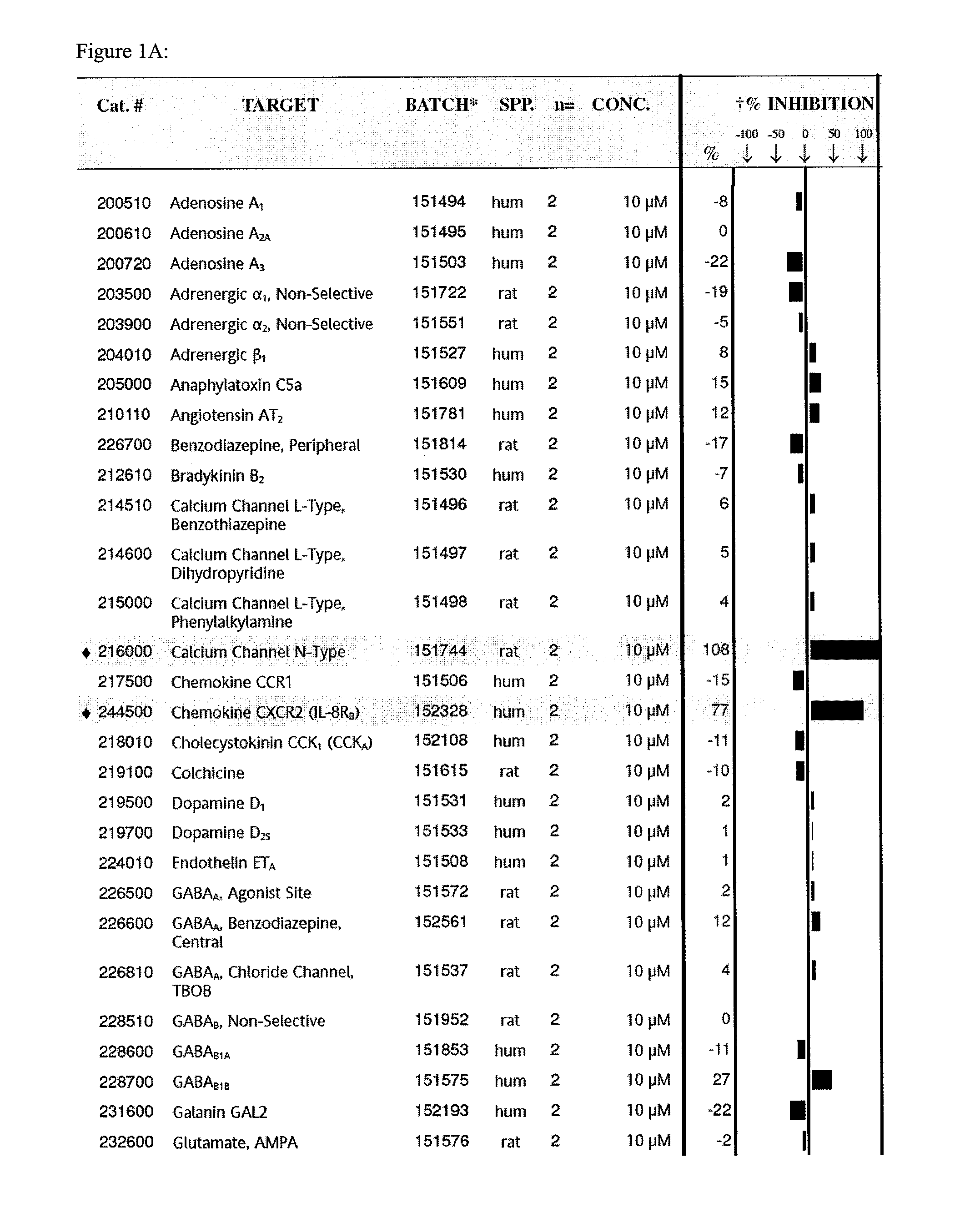
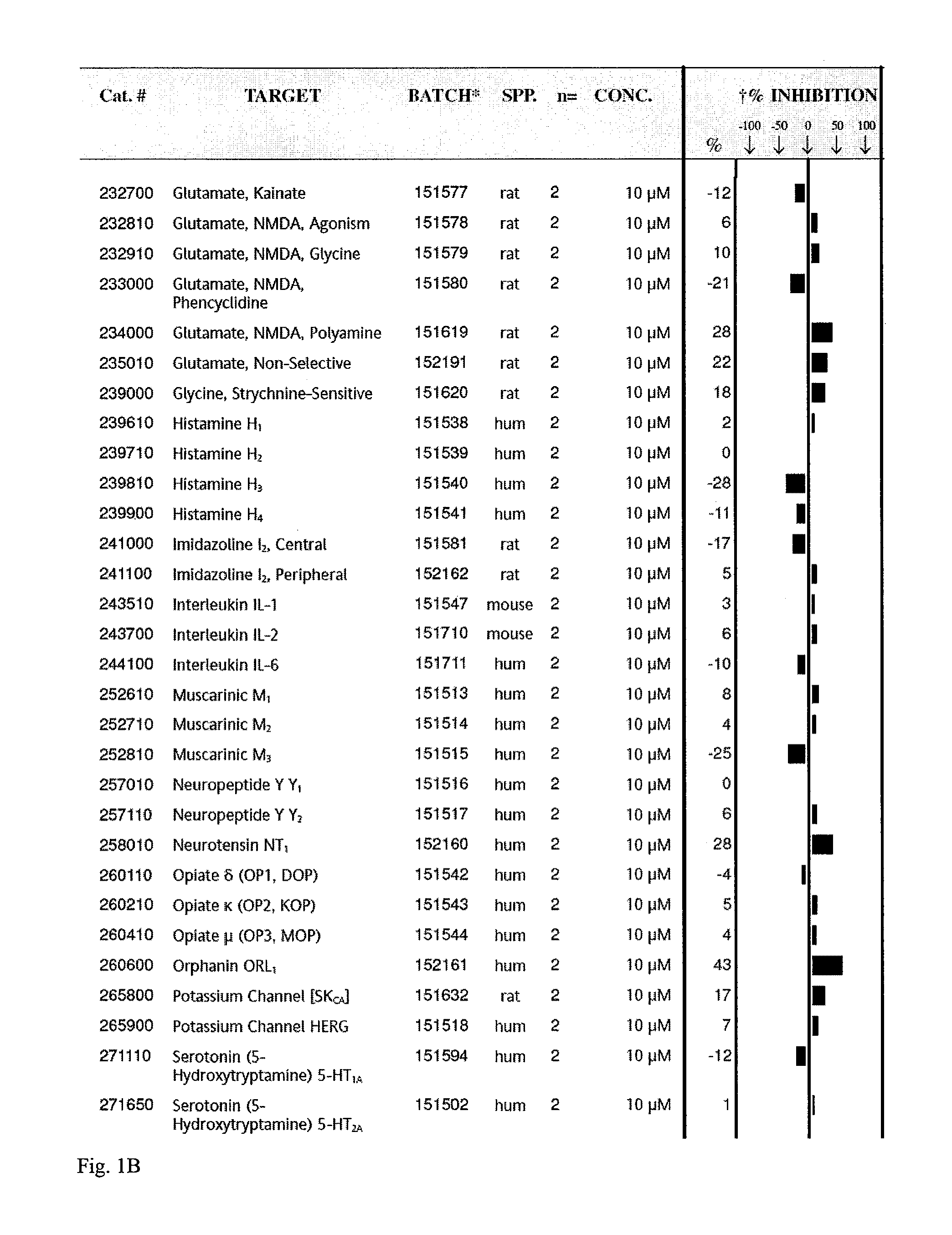
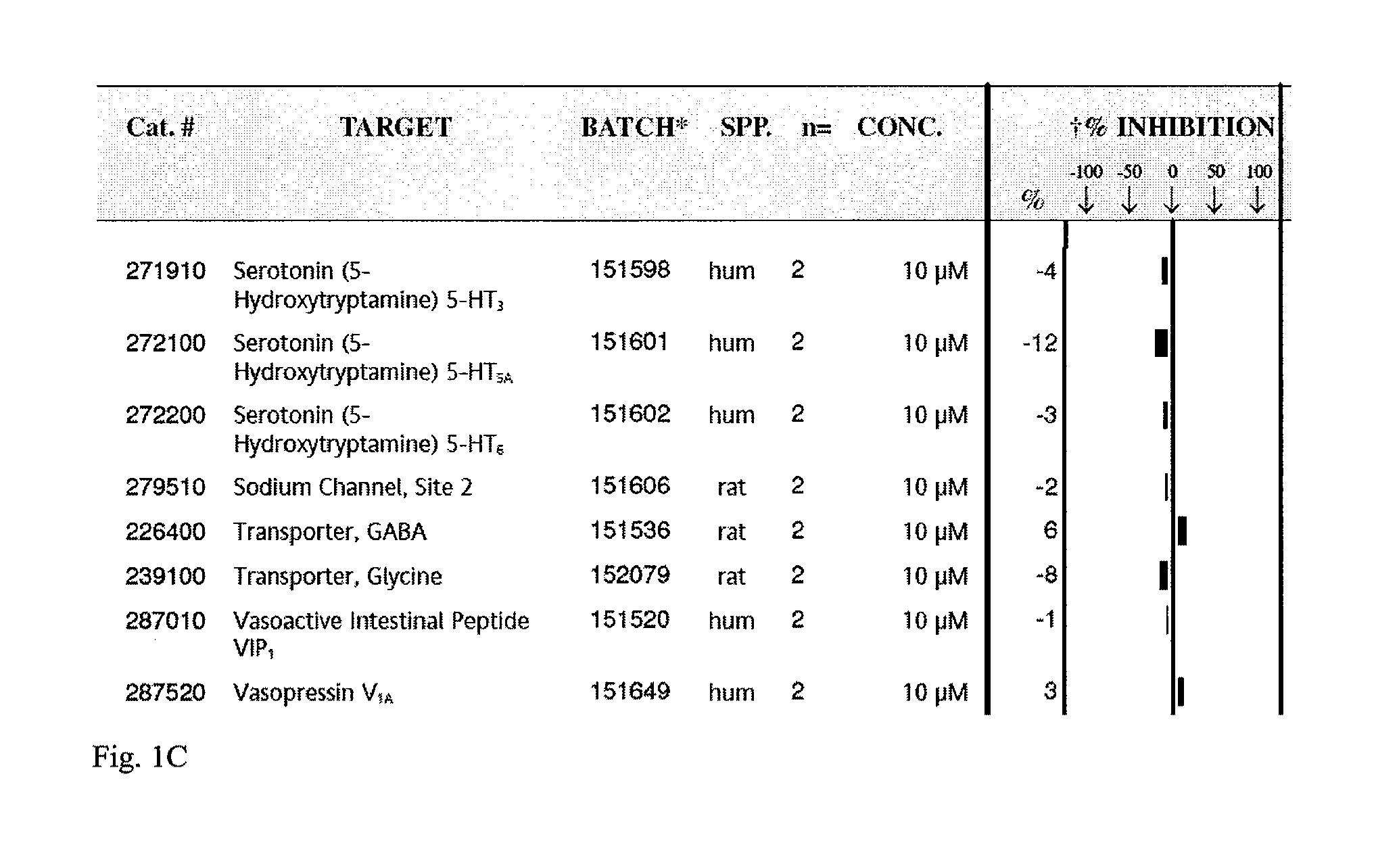
[0109]Tat-NR2B9c is a chimeric peptide of a standard tat peptide and KLSSIESDV (SEQ ID NO:12) previously shown to be effective in a rat model of stroke. This example screens the peptide Tat-NR2B9c for capacity to inhibit binding of known ligands to about 70 receptors proteins. Examples of receptors screened included glutamate, histamine H1, potassium channels, Dopamine D1, calcium channels (L-type, N-type).
[0110]Tat-NR2B9c was found to inhibit binding to two such receptors, an N-type calcium channel and a chemokine CXCR2 (IL-8Rb). The screen was performed as a competitive binding assay in which unlabelled Tat-NR2B9c at a concentration of 10 μM competed with an I125 labeled ligand for binding to its receptor in the presence of unlabeled ligand to increase sensitivity. At 10 μM, Tat-NR2B9c showed 100% inhibition of radiolabeled ω-Conotoxin GVIA binding to N-type Ca channels. Tat-NR2B9c also showed 80% inhibition of IL-8 / IL-8RB at the same concen...
example 2
Mutagenesis of a Standard Tat Peptide
[0111]Like the known N-type calcium channel inhibiter Ziconotide, Tat-NR2B9c contains numerous positive charges. The positive charges presumably facilitate both the ability to cross the blood brain barrier and may also contribute to N-type calcium channel binding. Direct sequence comparison shows some similarity in positive (R=Arginine, K=Lysine) charges as well as spacing of these charges along the peptide backbone (see alignment below). This approximately maps the Tat-NR2B9c N-type calcium channel binding epitope to the Tat region (shown in italics) and one amino acid of the NMDAR2B domain.
[0112]
(SEQ ID NO:17)YGRKKRRQRRRKLSSIESDV(Tat-NR2B9c)(SEQ ID NO:72)CKGKGAKCSRLMYDCCTGSCRSGKCG(Ziconotide)
[0113]The present inventors hypothesized that mutation of the Y residue at position 1 of Tat-NR2B9c to F might reduce binding to N-type calcium channels without impairing cellular uptake of the drug. The inventors also hypothesized that modifications of a s...
example 3
F-Tat-NR2B9c is Equally Effective in a Stroke Model
[0124]F-Tat-NR2B9c was compared with Tat-NR2B9c at a single does of 3 mmol / g weight in the rat pial occlusion model of permanent ischemia described above and further in example 4. In both cases, the chimeric peptide was administered one hour after initiating ischemia. F-Tat-NR2B9c and Tat-NR2B9c were equally effective in reducing infarct size as shown in FIG. 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| L-type currents | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com