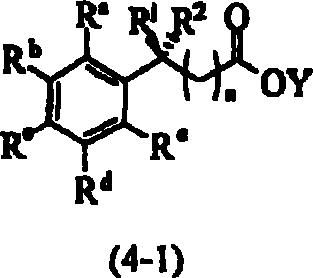
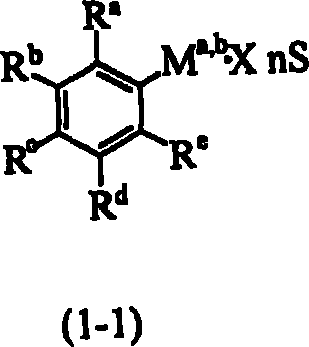
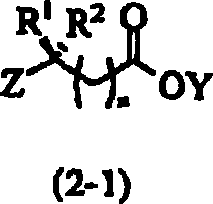Process for the preparation of fluorophenylalkylene acid derivatives
A technology for fluorophenyl alkylene carboxylic acid and derivatives, which is applied in the field of preparing fluorophenyl alkylene acid derivatives and can solve problems such as low yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] To a suspension of magnesium (0.132 moles, 3.2 g) in THF was added dropwise a solution of 1-bromo-3,4,5-trifluorobenzene (0.132 moles, 27.8 g) in THF. After the exothermic reaction had died down, the reaction mixture was refluxed for 30 minutes. To a stirred solution of anhydrous zinc chloride (0.132 mol, 18 g) in THF cooled to 0°C was added dropwise the Grignard solution. When the dropwise addition was complete, the solution was stirred at room temperature for an additional 30 minutes. The reaction mixture was cooled to 0°C, ethyl bromoacetate (0.11 mol, 18.4 g) and catalyst bis(acetylacetonate)-2,2'-bipyridine nickel(II) (0.4 mmol, 219 mg, 0.5 mol%) were added ). The reaction was kept at 5°C overnight. The solution was quenched with ice / water and extracted with ether. The organic layer was washed with saturated sodium chloride solution. After drying over sodium sulfate, the solvent was evaporated. The crude product was purified by distillation, isolated yield: 1...
Embodiment 2
[0104] To a stirred solution of 1,2-difluorobenzene in THF cooled to -78°C was added dropwise a solution of n-butyllithium (1.3 mmol) in hexane. After stirring at -78°C for 1 h, a solution of anhydrous zinc chloride (1.3 mmol) in THF was added dropwise, and the mixture was allowed to warm to room temperature. The solution was stirred at room temperature for 1 hour and cooled to 0 °C. Ethyl bromoacetate (1.3 mmol) and the catalyst bis(acetylacetonate)-2,2'-bipyridine nickel(II) (0.4 mmol / 0.5 mol%) were added, then the reaction was kept at room temperature overnight. The solution was quenched with ice / water and extracted with ether. The organic layer was washed with saturated sodium chloride solution. After drying over sodium sulfate, the solvent was evaporated. The crude product was purified by distillation. The yield was 77%.
Embodiment 3
[0106] According to the method of Example 2, ethyl 2-(2,3-difluorophenyl)acetate was produced from 1,2-difluorobenzene and ethyl bromoacetate. Different from Example 2, the molar ratio of aryl compound and ester was 1.5:1, and the reaction time was 1 hour. The yield was 74%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com