Slow release antibiotics preparation of bioactive substance
A technology of antibiotics and preparations, which is applied in the field of antibiotics preparations, and can solve the problems of release reaction impact, insignificance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
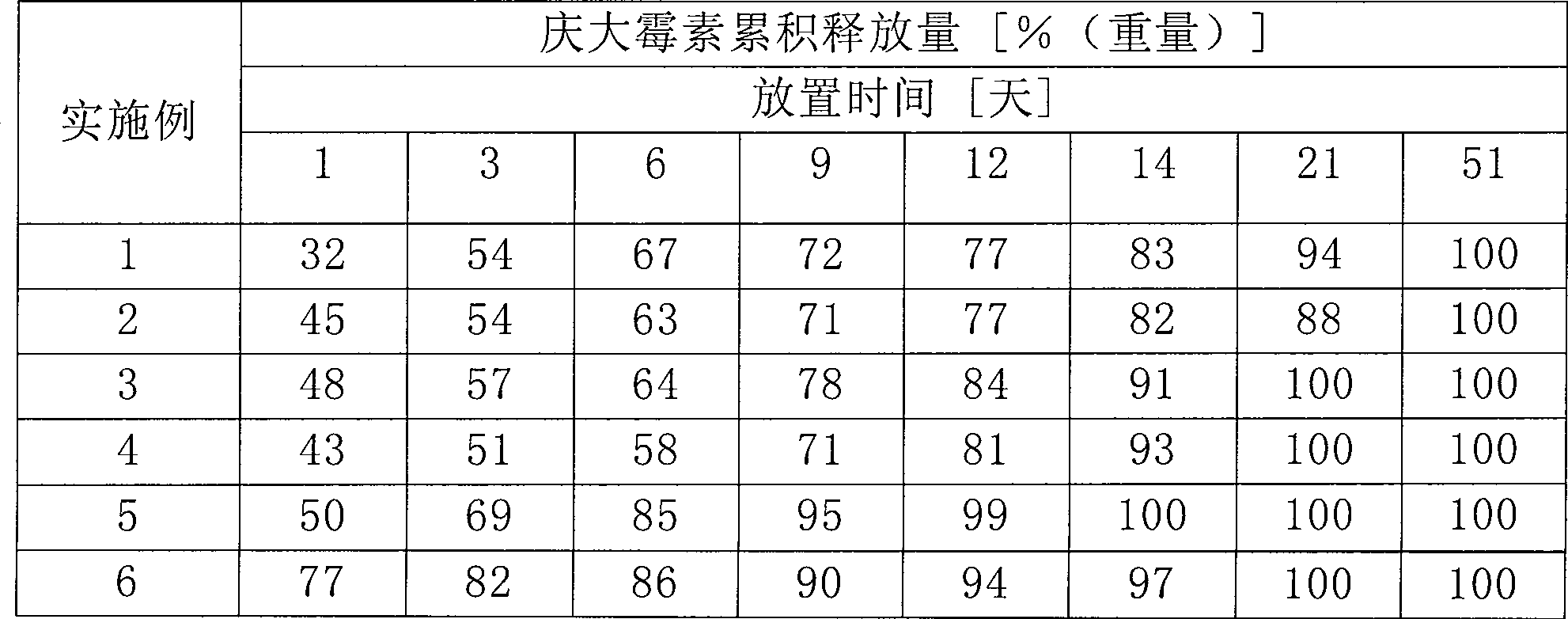
[0054] Prepare a kind of by 51mg gentamicin sulfate (700U / mg, Fluka company produces), 51mg sodium lauryl sulfate (Aldrich company produces), 280mg poly L-lactide (molecular weight~10,000gmol -1 ) and 1118mg of dibasic calcium phosphate (produced by Fluka). Each 200 mg of this mixture was pressed into a disc-shaped molding having a diameter of 13 mm with a press within 2 minutes under a pressure of 5 tons.
[0055] Wherein, the molar ratio of the amphoteric component and the antibiotic is >SDS:Genta=177:0.72=2.458333333:1
Embodiment 2
[0057] Prepare a kind of by 51mg gentamicin sulfate (700U / mg, Fluka company produces), 51mg sodium lauryl sulfate (Aldrich company produces), 280mg poly L-lactide (molecular weight~10,000gmol -1 ) and 1118mg of dibasic calcium phosphate dihydrate (produced by Fluka). Each 200 mg of this mixture was pressed into a disc-shaped molding having a diameter of 13 mm with a press within 2 minutes under a pressure of 5 tons.
[0058] Wherein, the molar ratio of the amphoteric component and the antibiotic is >SDS:Genta=177:0.72=2.458333333:1
Embodiment 3
[0060] Prepare a kind of by 51mg gentamicin sulfate (700U / mg, Fluka company produces), 51mg sodium lauryl sulfate (Aldrich company produces), 280mg poly L-lactide (molecular weight~10,000gmol -1 ) and 1118mg of calcium sulfate dihydrate (Fluka) mixture. Each 200 mg of this mixture was pressed into a disc-shaped molding having a diameter of 13 mm with a press within 2 minutes under a pressure of 5 tons.
[0061] Wherein, the molar ratio of the amphoteric component and the antibiotic is >SDS:Genta=177:0.72=2.458333333:1
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap