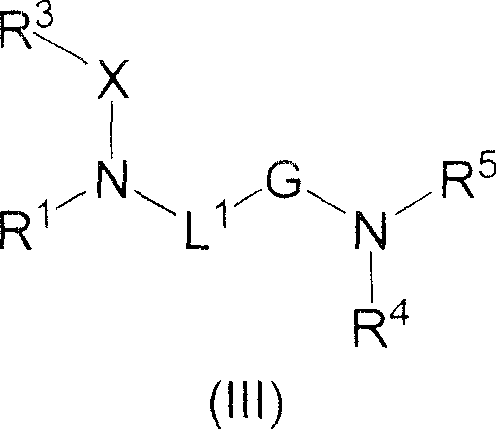
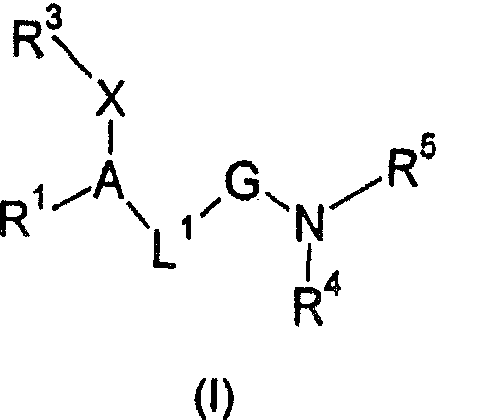
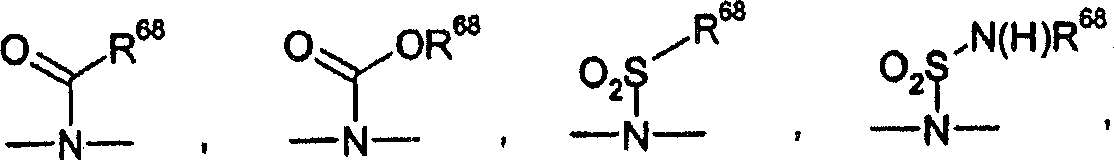
Amide derivatives as GK activators
A compound and substituent technology, applied in the field of amide derivatives as GK activators, can solve the problem that weight loss is not a therapeutic target
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0523] 2-(3,4-Dichlorophenoxy)-hexanoic acid thiazol-2-ylamide
[0524]
[0525] 2-(3,4-Dichlorophenoxy)hexyl was prepared from ethyl 2-hydroxyhexanoate (0.32 g, 2.0 mmol) and 3,4-dichlorophenol (0.39 g, 2.4 mmol) according to general procedure A Acid (0.3 g, 55%). A solution of this acid (69 mg, 0.25 mmol) in THF (3 ml) was reacted with 2-aminothiazole (60 mg, 0.6 mmol) according to general procedure E to give 2-(3,4-dichlorophenoxy)-hexyl Acid thiazol-2-ylamide (64 mg, 72%).
[0526] LCMS(m / z): 360(M+H) +
[0527] 1 H NMR (400MHz, CDCl 3 ): δ 0.89(t, 3H), 1.30-1.51(m, 4H), 2.02(m, 2H), 4.73(t, 1H), 6.76(dd, 1H), 7.03(dd, 2H), 7.33(d , 1H), 7.49(d, 1H), 9.98(br, 1H).
Embodiment 2
[0529] 2-(4-fluorophenoxy)-N-1,3-thiazol-2-ylhexanamide
[0530]
[0531] 2-(4-Fluorophenoxy)hexanoic acid (0.23 g, 52 %). A solution of this acid (56 mg, 0.25 mmol) in THF (3 ml) was reacted with 2-aminothiazole (60 mg, 0.6 mmol) following general procedure E to afford 2-(4-fluorophenoxy)-N-1, 3-Thiazol-2-ylhexanamide (58 mg, 76%).
[0532] LCMS (m / z): 309 (M+H) +
[0533] 1 H NMR (400MHz, CDCl 3): δ 0.89(t, 3H), 1.32-1.50(m, 4H), 1.99(m, 2H), 4.69(t, 1H), 6.81-6.84(m, 2H), 6.94-7.02(m, 3H) , 7.50(d, 1H), 10.24(br, 1H).
Embodiment 3
[0535] 2-(4-Methoxyphenoxy)-N-1,3-thiazol-2-ylhexanamide
[0536]
[0537] 2-(4-Methoxyphenoxy)hexanoic acid ( 0.23 g, 48%). A solution of this acid (60 mg, 0.25) in THF (3 ml) was reacted with 2-aminothiazole (60 mg, 0.6 mmol) via Method E to afford 2-(4-methoxyphenoxy)-N-1 , 3-thiazol-2-ylhexanamide (130 mg, 82%).
[0538] LCMS (m / z): 321 (M+H) +
[0539] 1 H NMR (400MHz, CDCl 3 ): δ0.89(t, 3H), 1.31-1.52(m, 4H), 1.98(m, 2H), 3.74(s, 3H), 4.67(t, 1H), 6.82(m, 4H), 7.01( d, 1H), 7.52 (d, 1H), 10.33 (br, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com