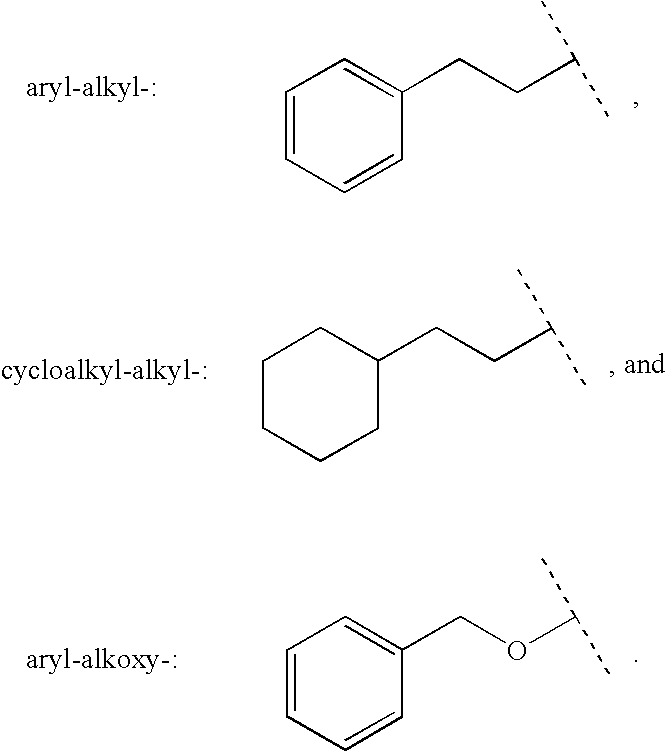
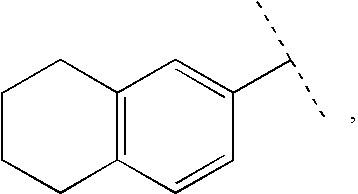
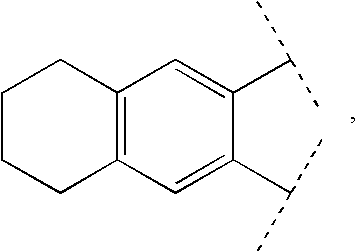
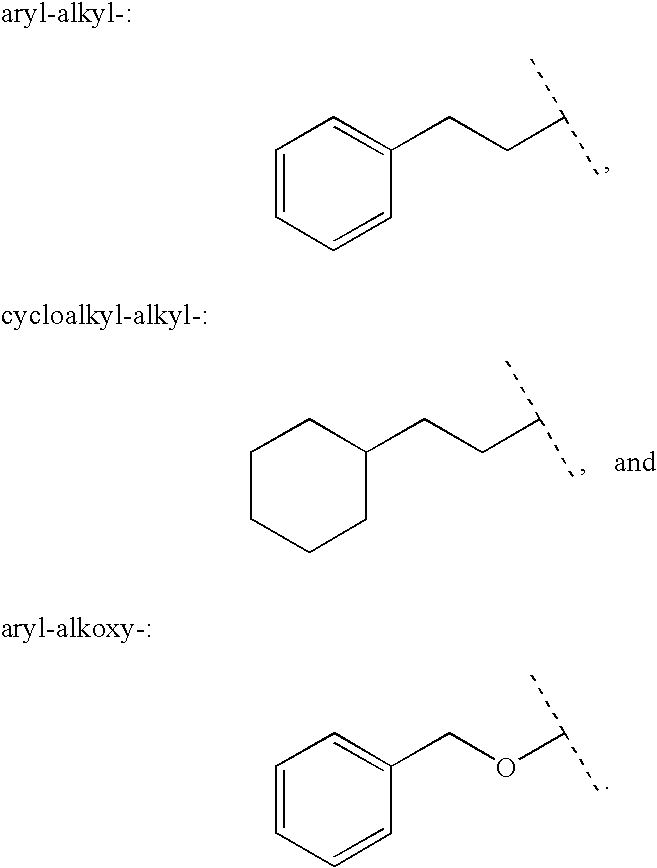
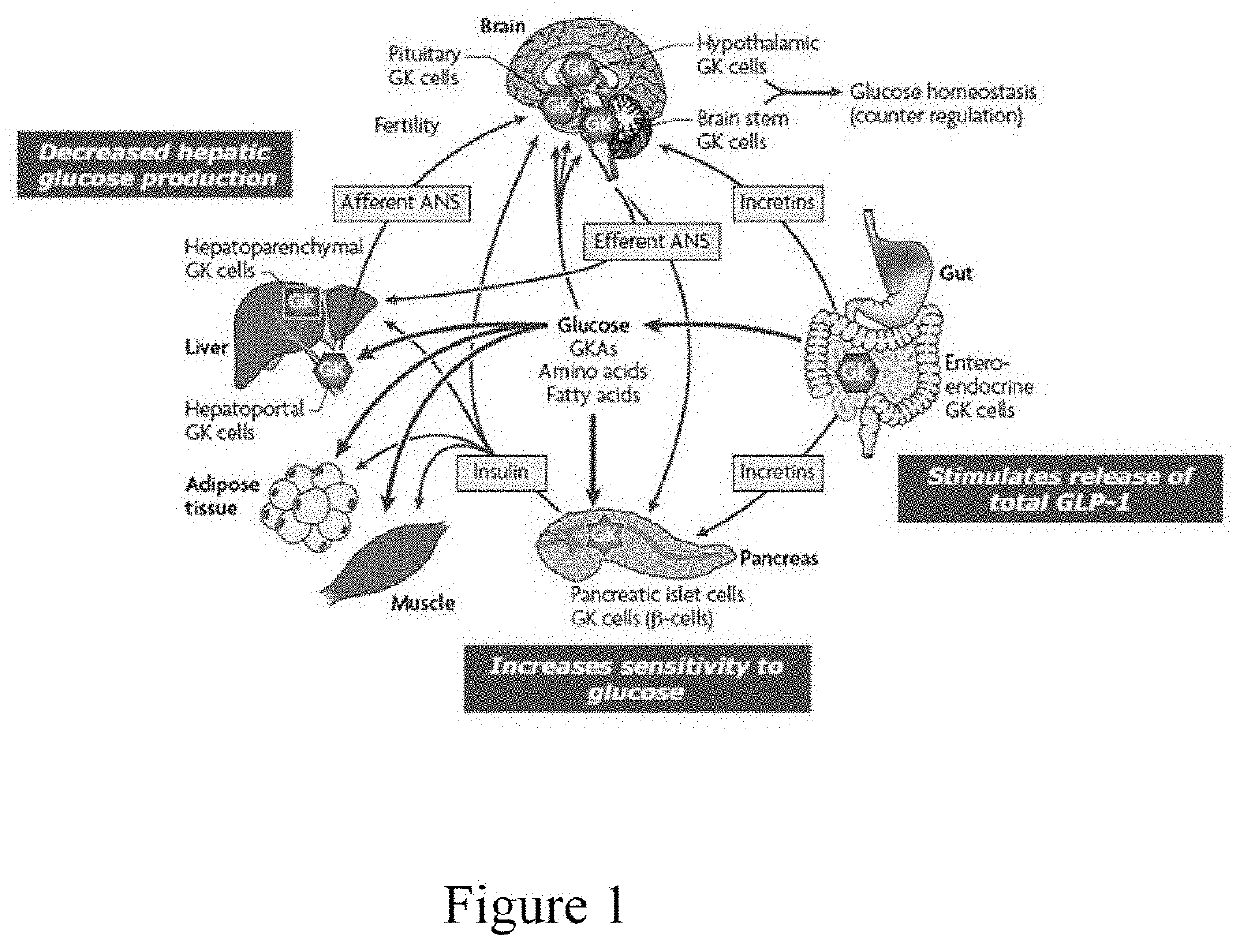
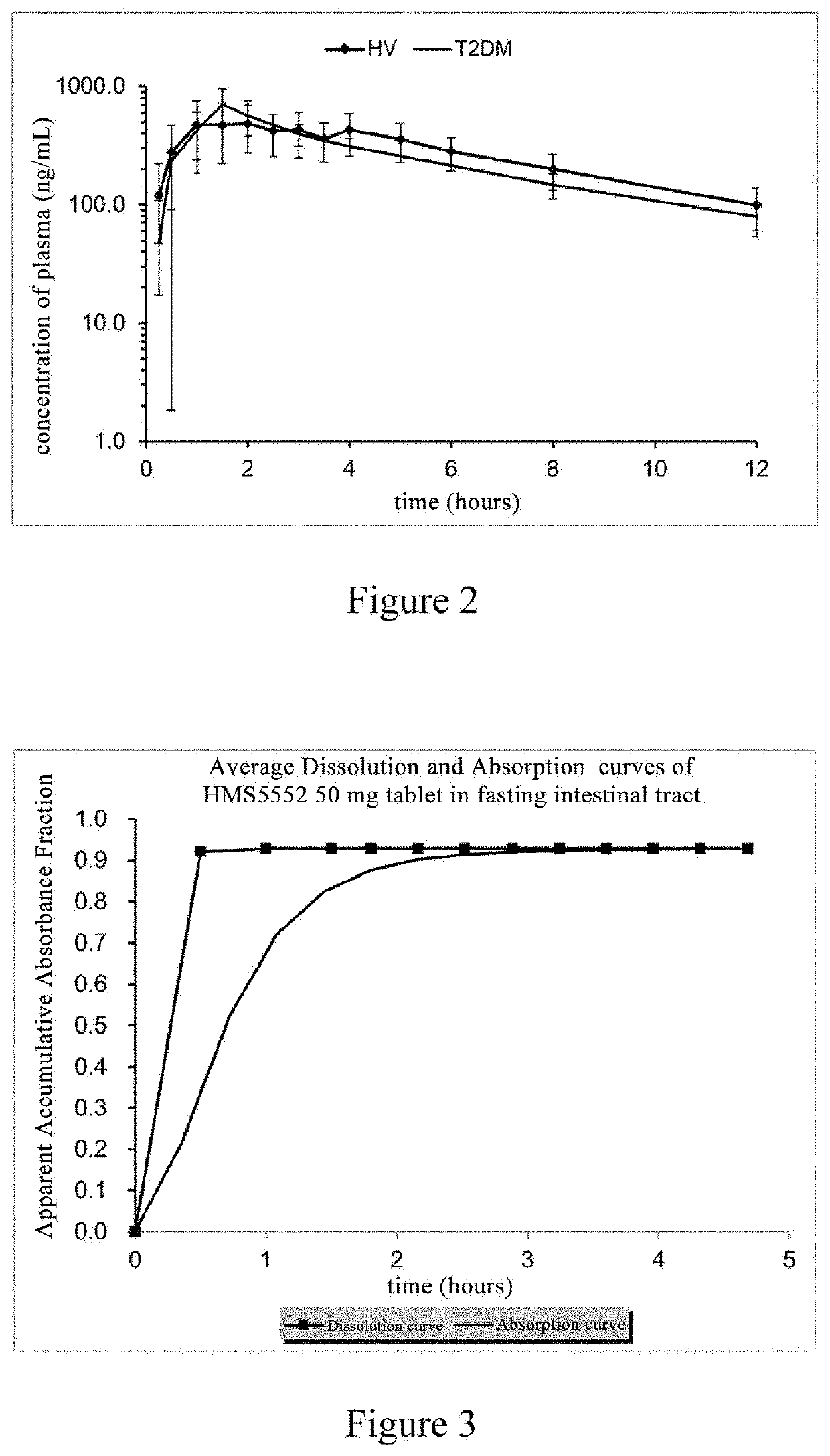
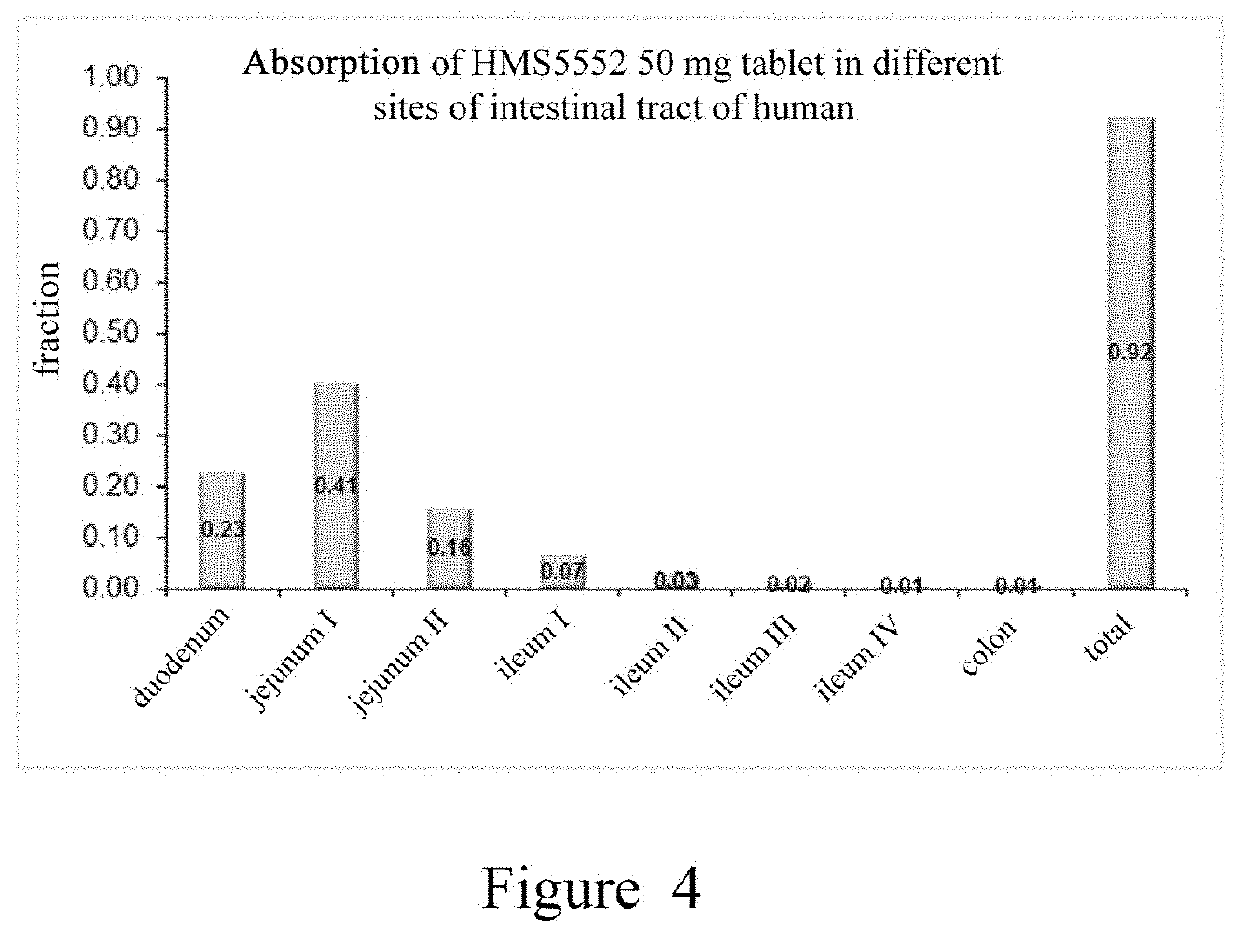
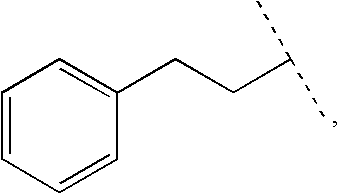
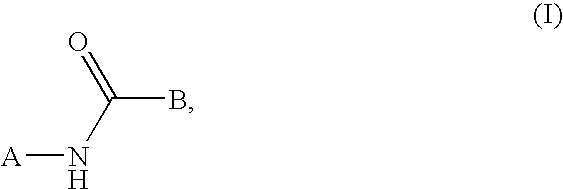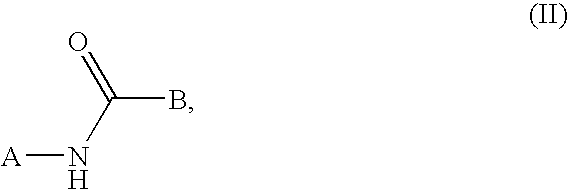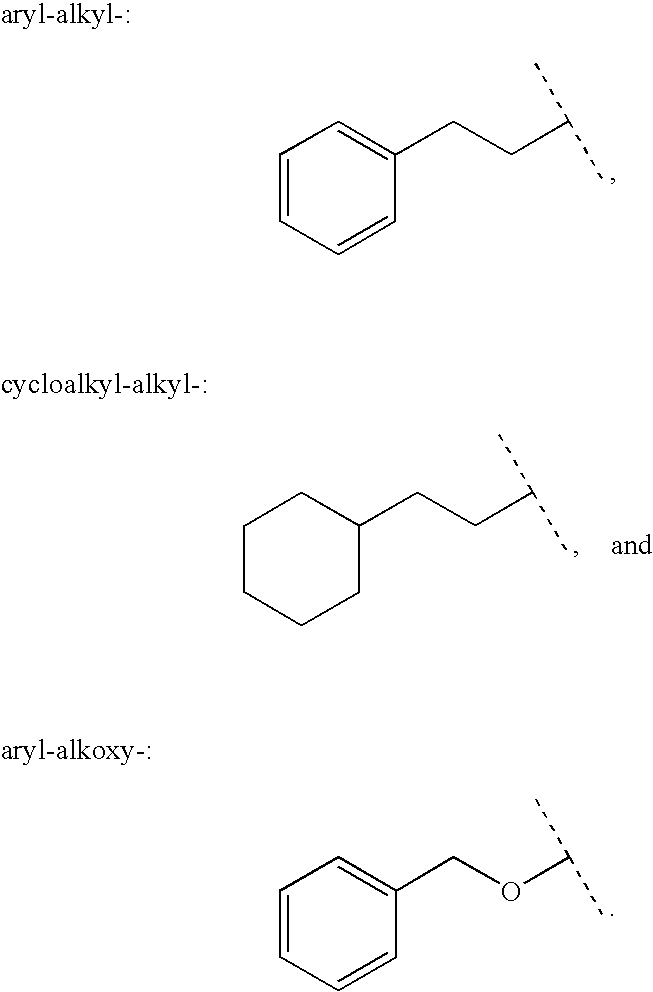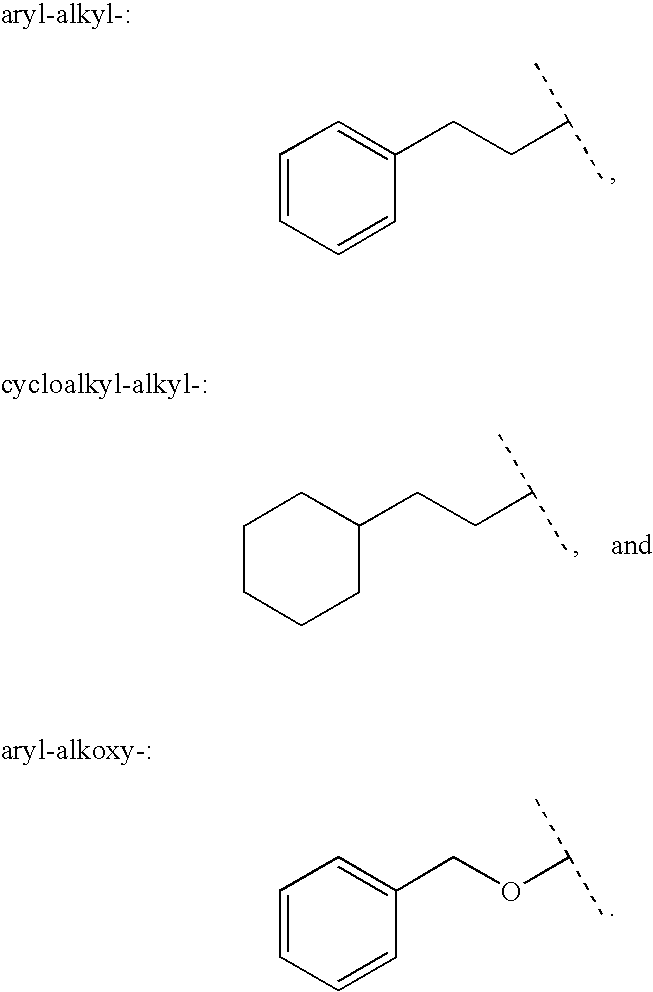Patents
Literature
59 results about "Glucokinase activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
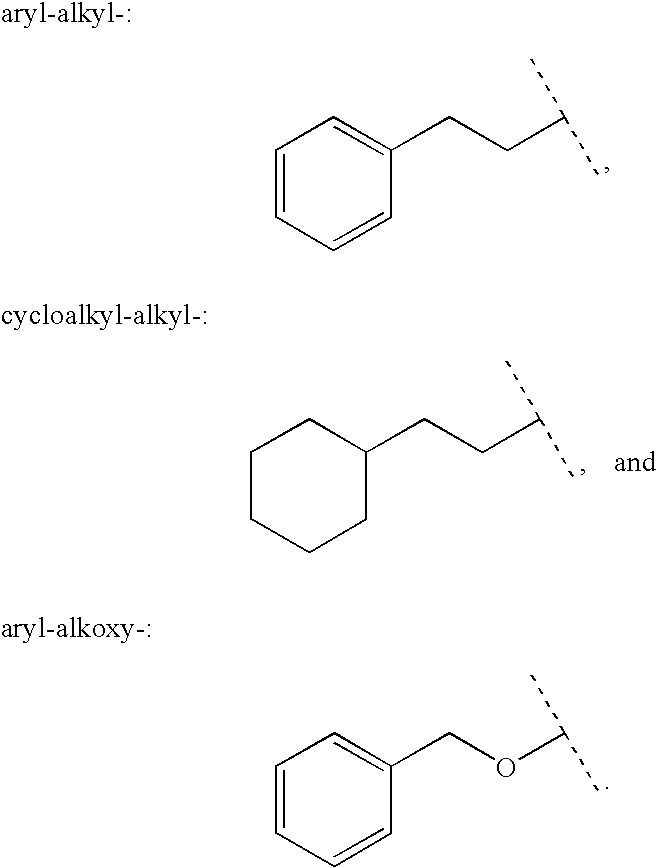
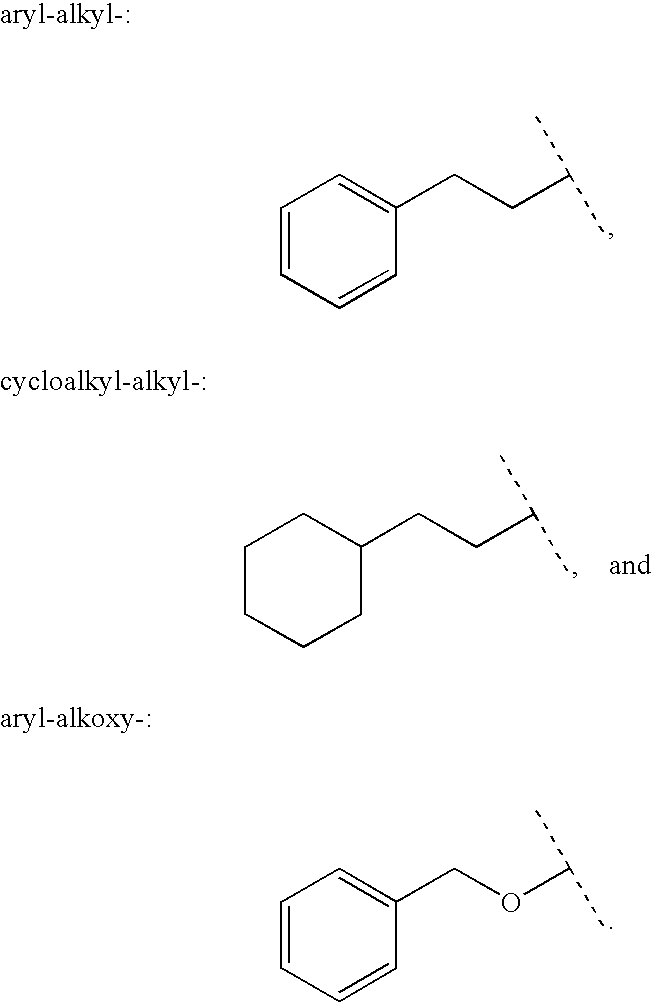
Aryl carbonyl derivatives as therapeutic agents
InactiveUS7384967B2Increase in number and sizeBiocideGroup 4/14 element organic compoundsArylBiochemistry
This invention relates to aryl carbonyl derivatives which are activators of glucokinase which may be useful for the management, treatment, control, or adjunct treatment of diseases, where increasing glucokinase activity is beneficial.
Owner:VTV THERAPEUTICS LLC
Aryl carbonyl derivatives as therapeutic agents
Owner:VTV THERAPEUTICS LLC
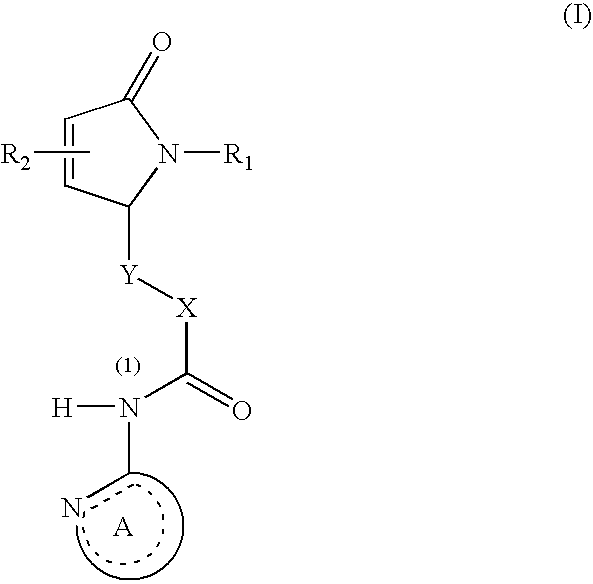
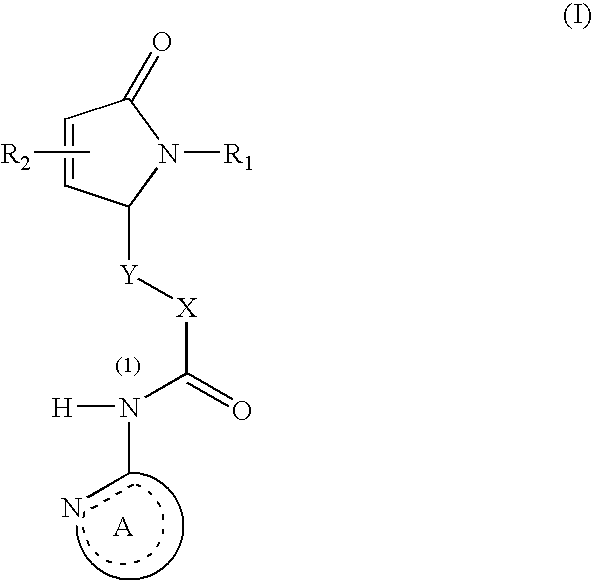
N-heteroaryl indole carboxamides and analogues thereof, for use as glcokinase activators in the treatment of diabetes
InactiveUS20070027140A1Establish and weight lossBiocideOrganic chemistryGlucokinase activityCancer research
This invention relates to compounds that are activators of glucokinase and thus may be useful for the management, treatment, control, or adjunct treatment of diseases, where increasing glucokinase activity is beneficial. The compounds are of the general formula (I) wherin A and B are further defined in the application.
Owner:TRANSTECH PHARMA
N-heteroaryl indole carboxamides and analogues thereof, for use as glucokinase activators in the treatment of diabetes
This invention relates to compounds that are activators of glucokinase and thus may be useful for the management, treatment, control, or adjunct treatment of diseases, where increasing glucokinase activity is beneficial. The compounds are of the general formula (I)wherein A and B are further defined in the application.
Owner:TRANSTECH PHARMA INC
Aryl carbonyl derivatives as therapeutic agents
ActiveUS20060183783A1Interfere with biological activityIncrease in number and sizeOrganic active ingredientsBiocideDiseaseAryl
This invention relates to aryl carbonyl derivativeswhich are activators of glucokinase which may be useful for the management, treatment, control, or adjunct treatment of diseases, where increasing glucokinase activity is beneficial.
Owner:VTV THERAPEUTICS LLC
Aryl carbonyl derivatives as therapeutic agents
InactiveUS20080119455A1Increase in number and sizeGroup 4/14 element organic compoundsBiocideArylBiochemistry
This invention relates to aryl carbonyl derivatives which are activators of glucokinase which may be useful for the management, treatment, control, or adjunct treatment of diseases, where increasing glucokinase activity is beneficial.
Owner:VTV THERAPEUTICS LLC
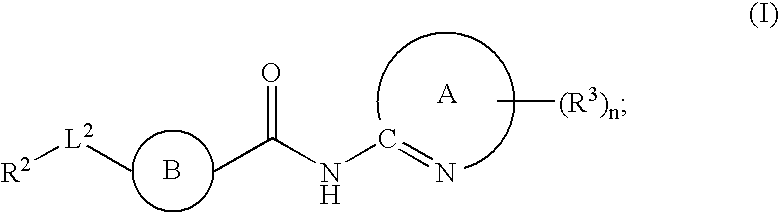
Fused phenyl amido heterocyclic compounds
ActiveUS20080280875A1Lower blood sugar levelsEffective compoundBiocideOrganic chemistryBenzeneChemical compound
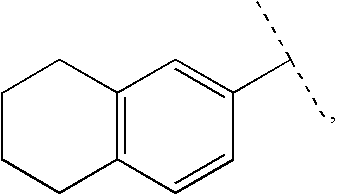
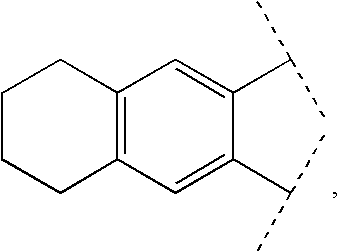
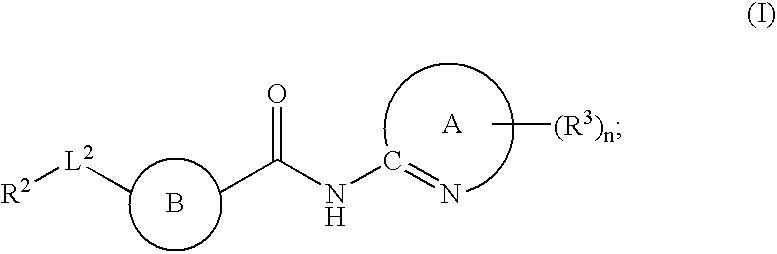
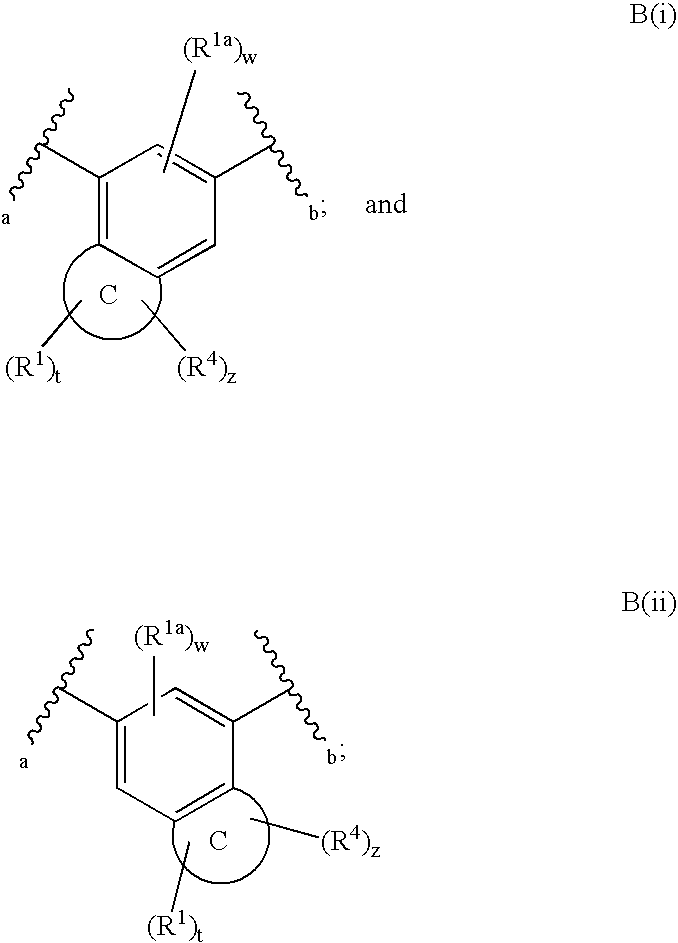
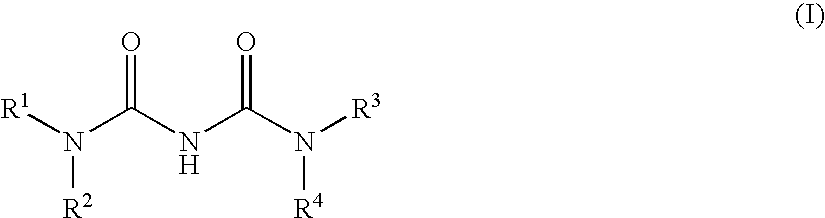
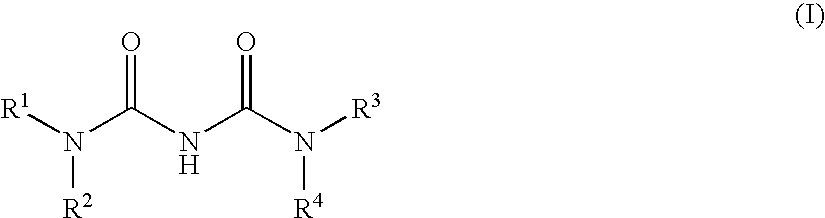
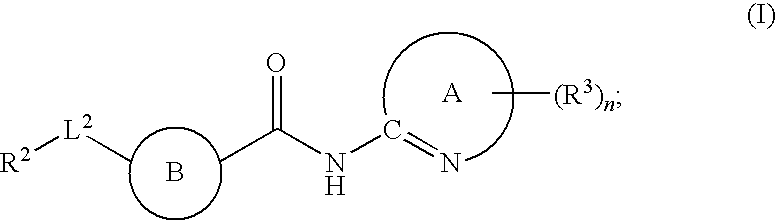
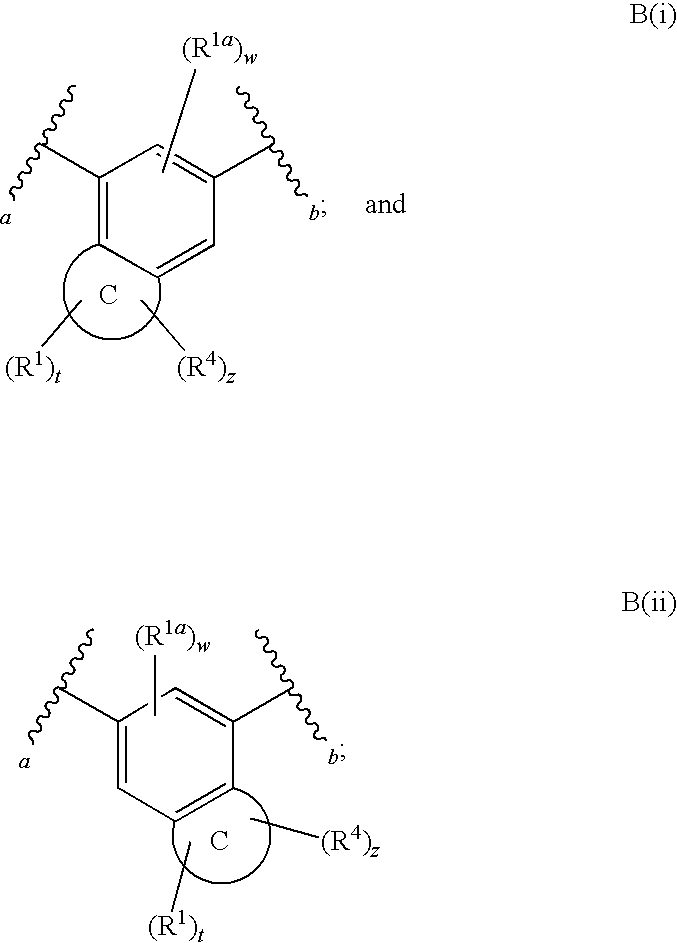
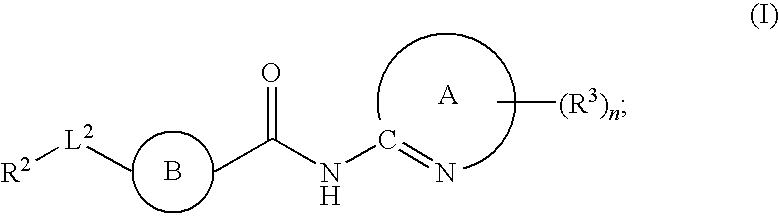
The present invention relates to a compound of formula (I):or a pharmaceutically acceptable salt or solvate thereof, wherein:Ring A is (4-12)-membered heterocyclyl;Ring B is a fused benzene ring selected from the group consisting of:Ring A, ring B, ring C, R1, R1a, R2, R3, R4, L2, n, t, w, and z are as defined in the specification. The invention also relates to pharmaceutical compositions comprising the compounds of formula (I) and methods of treating a condition that is mediated by the modulation of glucokinase, the method comprising administering to a mammal an effective amount of a compound of formula (I).
Owner:PFIZER INC
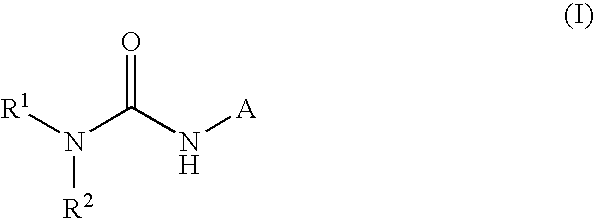
Heteroaryl-ureas and their use as glucokinase activators
This invention relates to compounds of formula (I) which are activators of glucokinase and thus may be useful for the management, treatment, control, or adjunct treatment of diseases, where increasing glucokinase activity is beneficial.
Owner:VTV THERAPEUTICS LLC
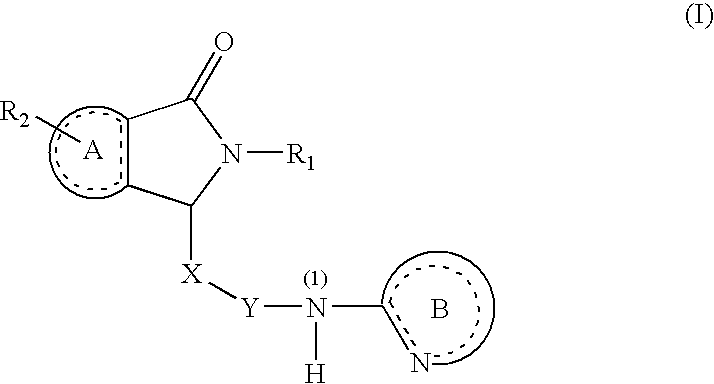
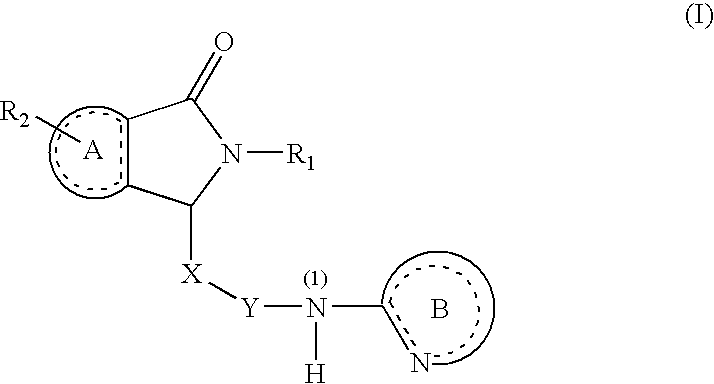
Substituted Dihydroisoindolones As Allosteric Modulators of Glucokinase
The present invention relates to compounds of Formula (I), methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating glucokinase mediated disorders. More particularly, the compounds of the present invention are glucokinase modulators useful for treating disorders including, but not limited to, type II diabetes.
Owner:JANSSEN PHARMA NV
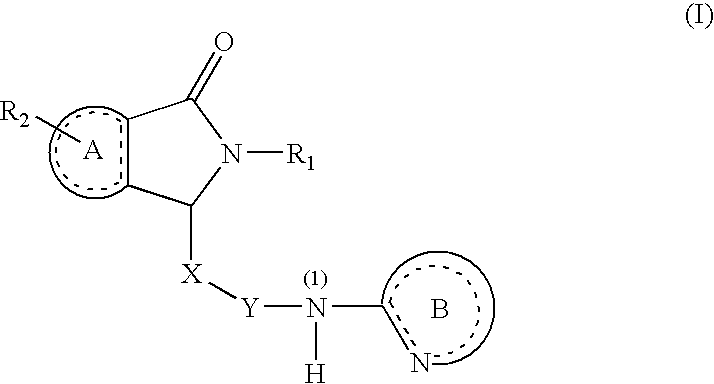
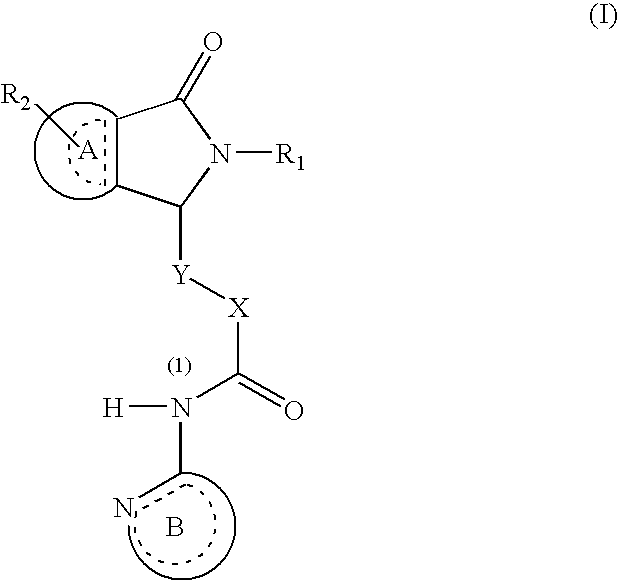
Dihydroisoindolones As Allosteric Modulators Of Glucokinase
The present invention relates to compounds of Formula (I), methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating glucokinase mediated disorders. More particularly, the compounds of the present invention are glucokinase modulators useful for treating disorders including, but not limited to, type II diabetes.
Owner:JANSSEN PHARMA NV
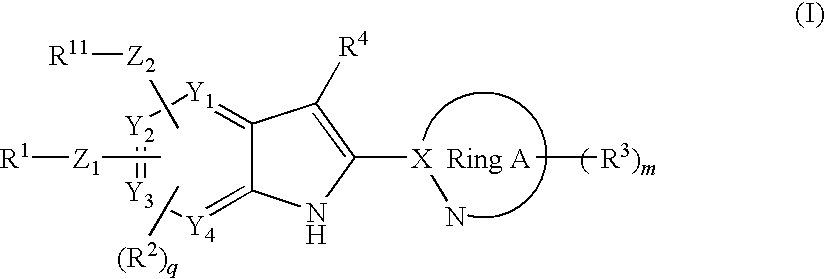
2-heteroaryl-substituted indole derivative
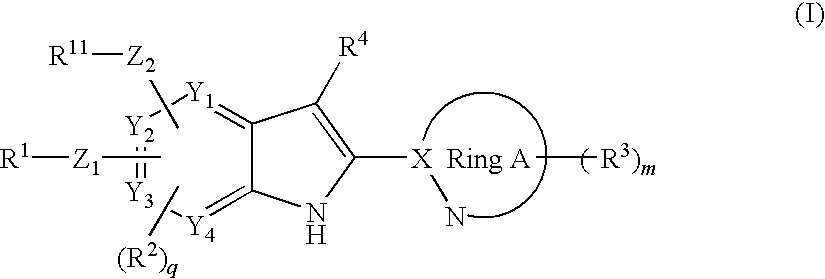
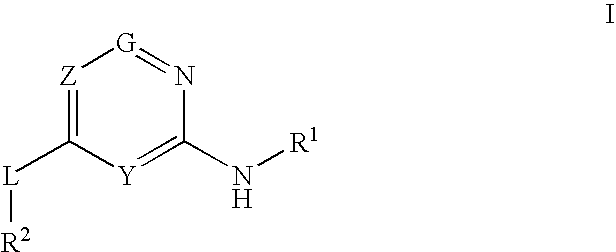
Disclosed is a compound represented by the formula (I) below, which has a glucokinase-activating effect and is thus useful for treatment of diabetes or obesity, or a pharmaceutically acceptable salt thereof.In the formula, R1 represents an aryl or the like; R11 represents an aryl or the like; R2 represents a formyl or the like, R3 represents a C1-6 alkyl or the like; R3 represents a hydrogen atom or the like; Z1 represents —O— or the like; Z2 represents —O— or the like; Y1-Y4 respectively represent a carbon atom or a nitrogen atom; ring A represents a heteroaryl group; X represents a carbon atom or the like; m represents an integer of 0-2; and q represents an integer of 0-2.
Owner:MSD KK
Heteroaryl-Ureas and Their Use as Glucokinase Activators
This invention relates to compounds of formula (I)which are activators of glucokinase and thus may be useful for the management, treatment, control, or adjunct treatment of diseases, where increasing glucokinase activity is beneficial.
Owner:NOVO NORDISK AS
Dicycloalkyl Urea Glucokinase Activators
Dicycloalkyl urea glucokinase activators compounds are glucokinase inhibitors useful for the treatment of diabetes.
Owner:VTV THERAPEUTICS LLC
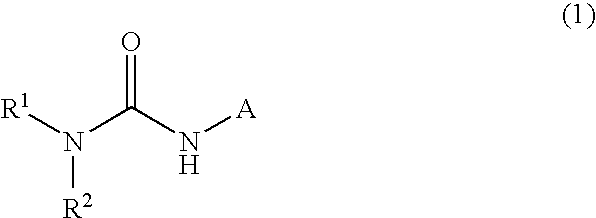
Dicycloalkylcarbamoyl Ureas As Glucokinase Activators
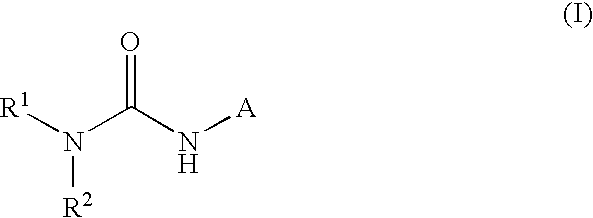
This invention relates to dicycloalkylcarbamoyl ureas of formula (I), which are activators of glucokinase and thus may be useful for the management, treatment, control, or adjunct treatment of diseases, where increasing glucokinase activity is beneficial.
Owner:TRANSTECH PHARMA
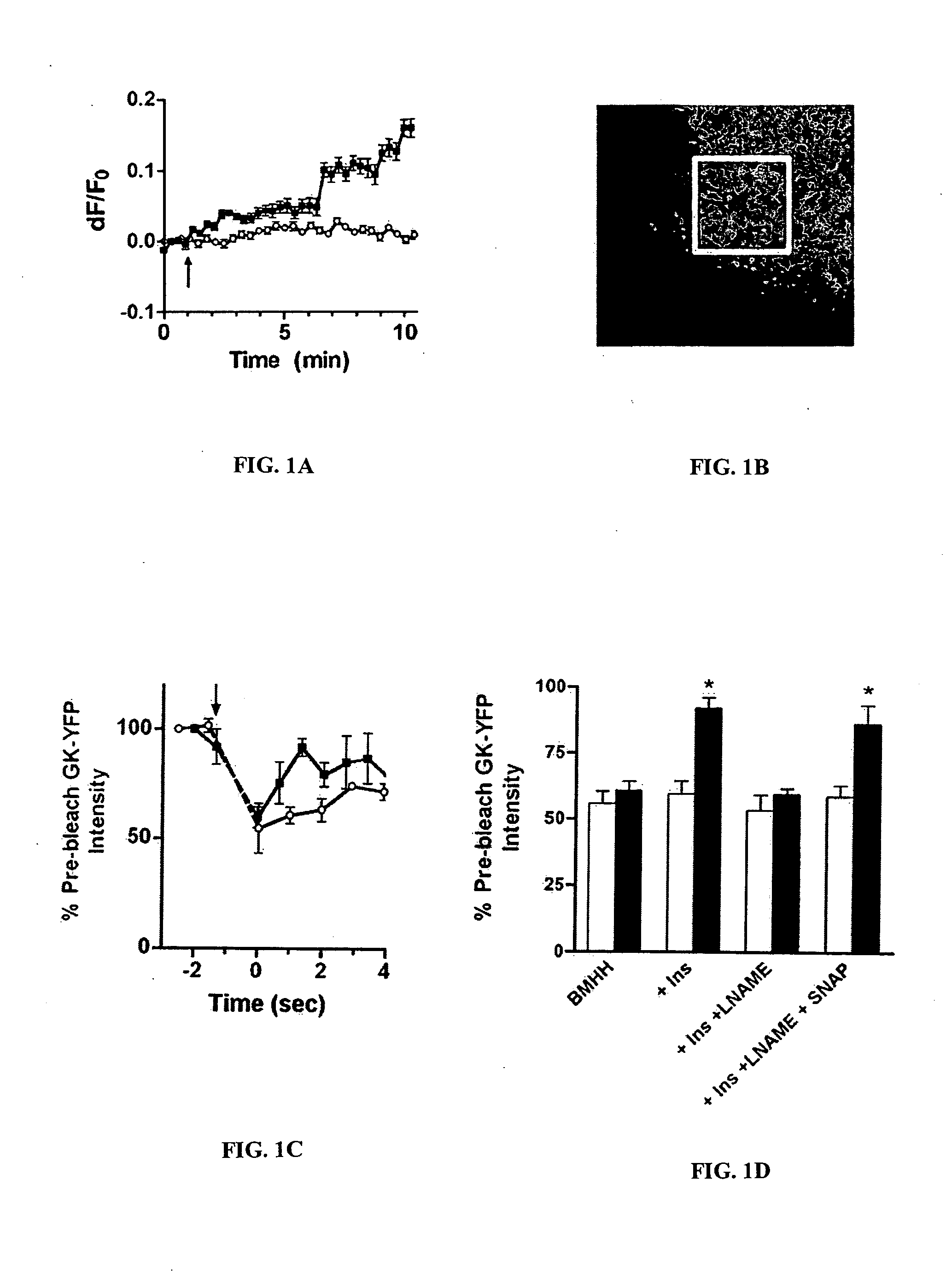
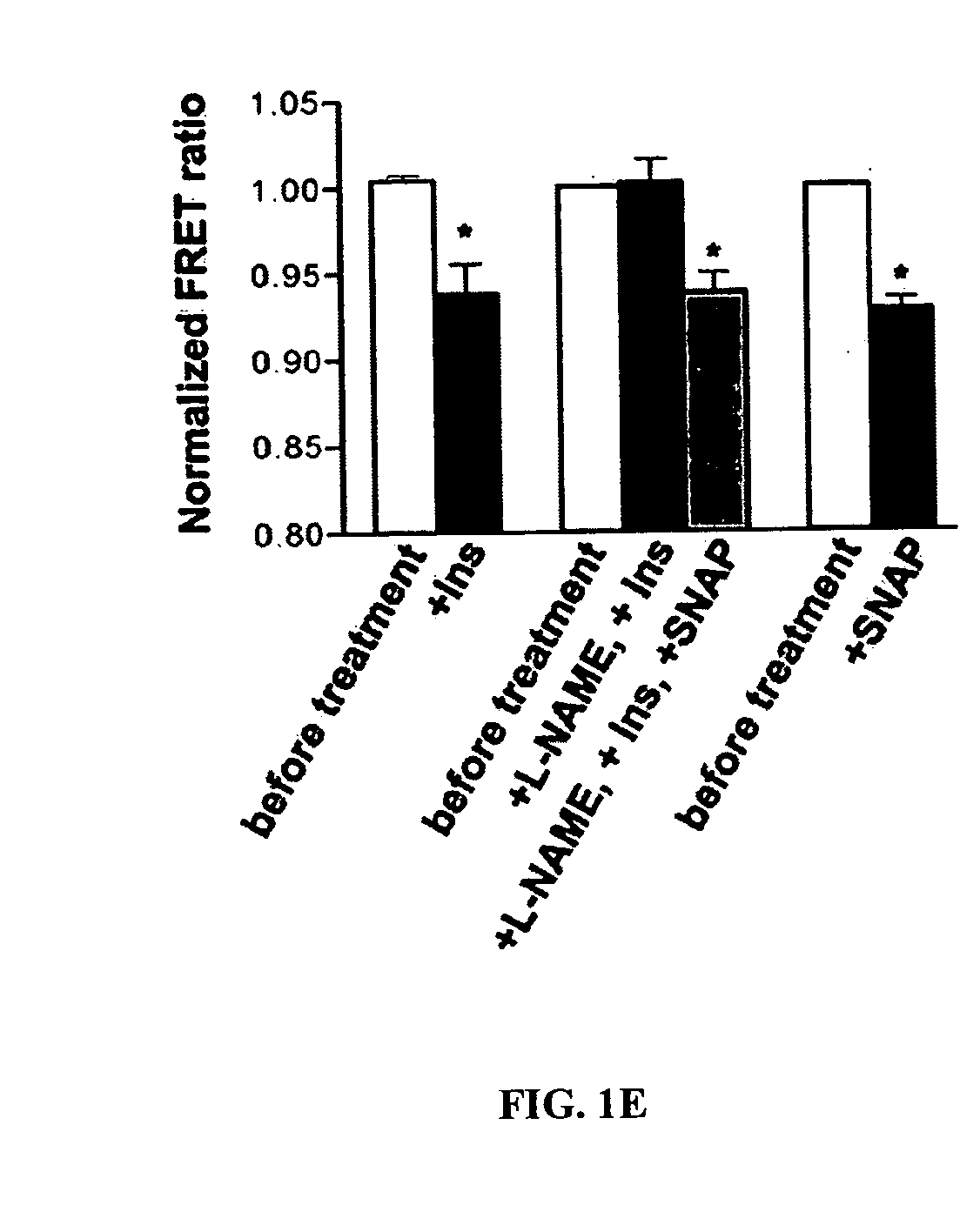
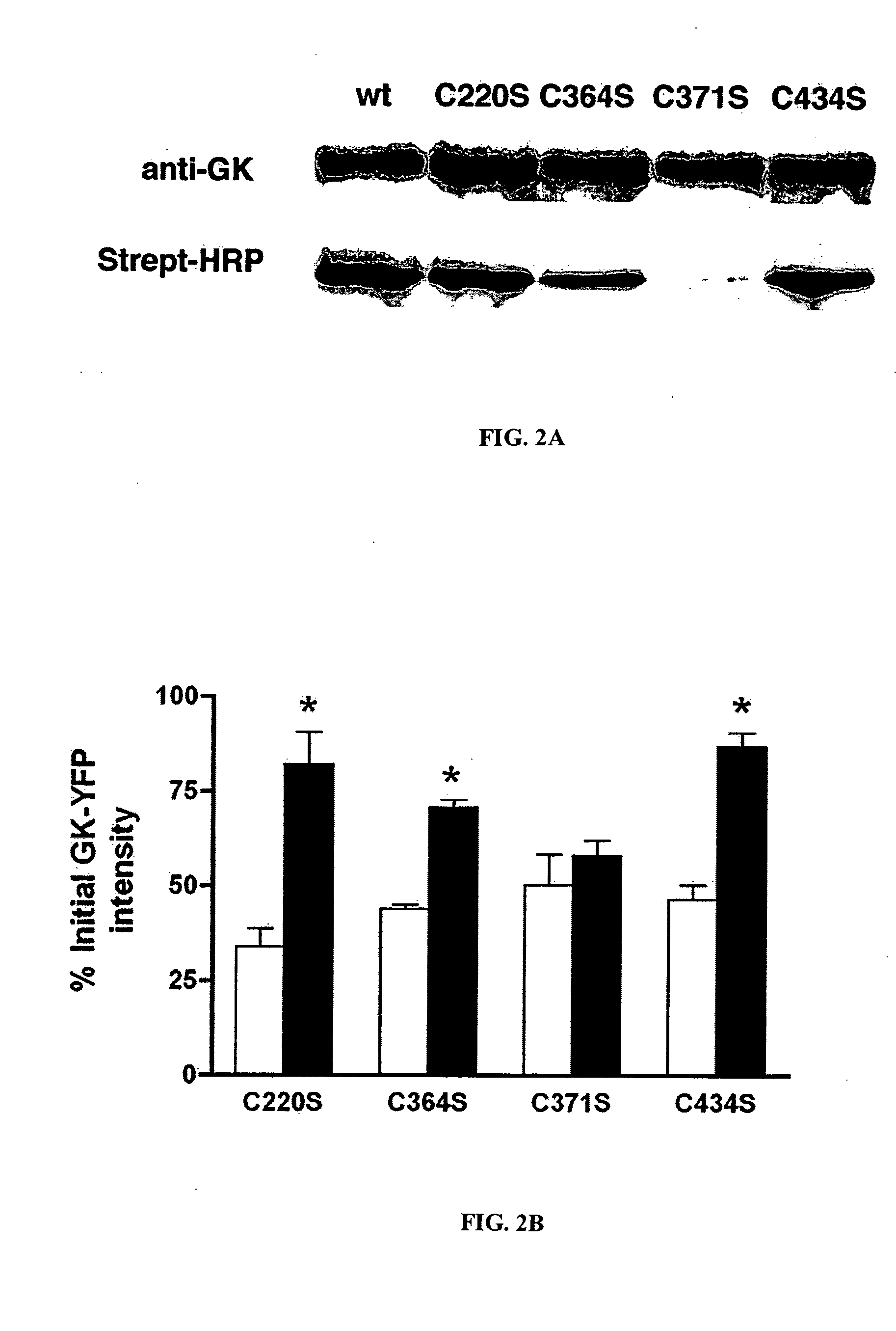
Methods of screening for a candidate modulator of glucokinase
The present invention relates to providing novel therapeutics for treating diabetes other glycemic disorders. Such therapeutics involve the signaling pathways that contribute to regulation of glucose-stimulated insulin secretion. Of particular interest are modulators of a key component in the glucokinase pathway. Thus, the present provides methods of screening for modulators of glucokinase activity, expression, translocation, conformation, nitrosylation and interaction with other molecules as useful target for pharmacological manipulation in the treatment of diabetes and other glycemic disorders.
Owner:VANDERBILT UNIV
Fused phenyl amido heterocyclic compounds
ActiveUS7842713B2Lower blood sugar levelsEase of preparation and detectabilityBiocideOrganic chemistryBenzeneChemical compound
The present invention relates to a compound of formula (I):or a pharmaceutically acceptable salt or solvate thereof, wherein:Ring A is (4-12)-membered heterocyclyl;Ring B is a fused benzene ring selected from the group consisting of:Ring A, ring B, ring C, R1, R1a, R2, R3, R4, L2, n, t, w, and z are as defined in the specification. The invention also relates to pharmaceutical compositions comprising the compounds of formula (I) and methods of treating a condition that is mediated by the modulation of glucokinase, the method comprising administering to a mammal an effective amount of a compound of formula (I).
Owner:PFIZER INC
Aryl carbonyl derivatives as therapeutic agents
This invention relates to aryl carbonyl derivatives which are activators of glucokinase which may be useful for the management, treatment, control, or adjunct treatment of diseases, where increasing glucokinase activity is beneficial.
Owner:VTV THERAPEUTICS LLC
Amide derivatives as GK activators
The present invention relates to compounds of general formula (I), which are activators of glucokinase (GK), and which may be suitable for the treatment, treatment, control and auxiliary treatment of such diseases: wherein increasing the activity of glucokinase is Beneficial, such as IGT, syndrome X, type 2 diabetes, type 1 diabetes, dyslipidemia, hyperlipidemia, hypertension and obesity.
Owner:NOVO NORDISK AS
Alpha functionalization of cyclic, ketalized ketones and products therefrom
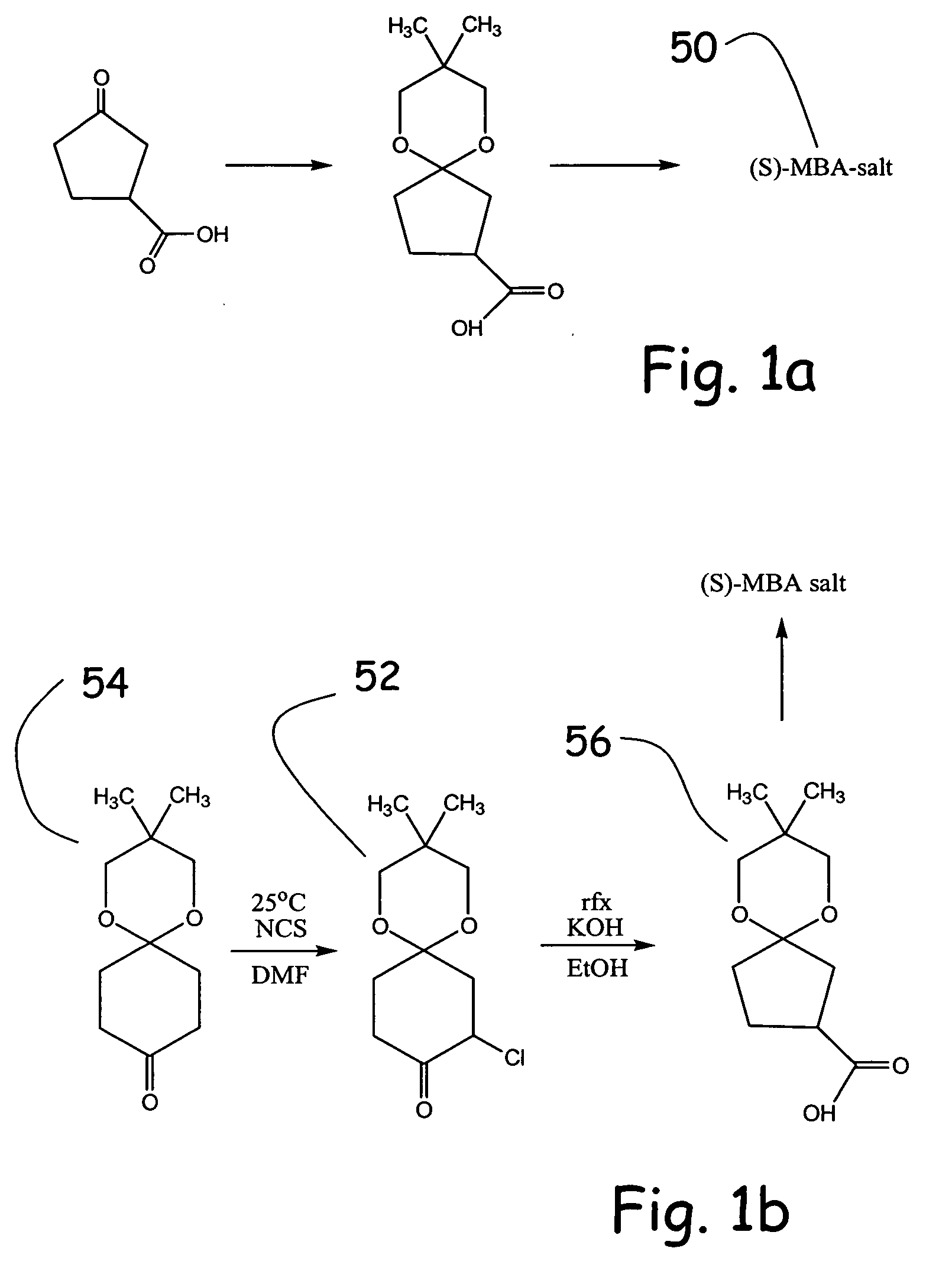
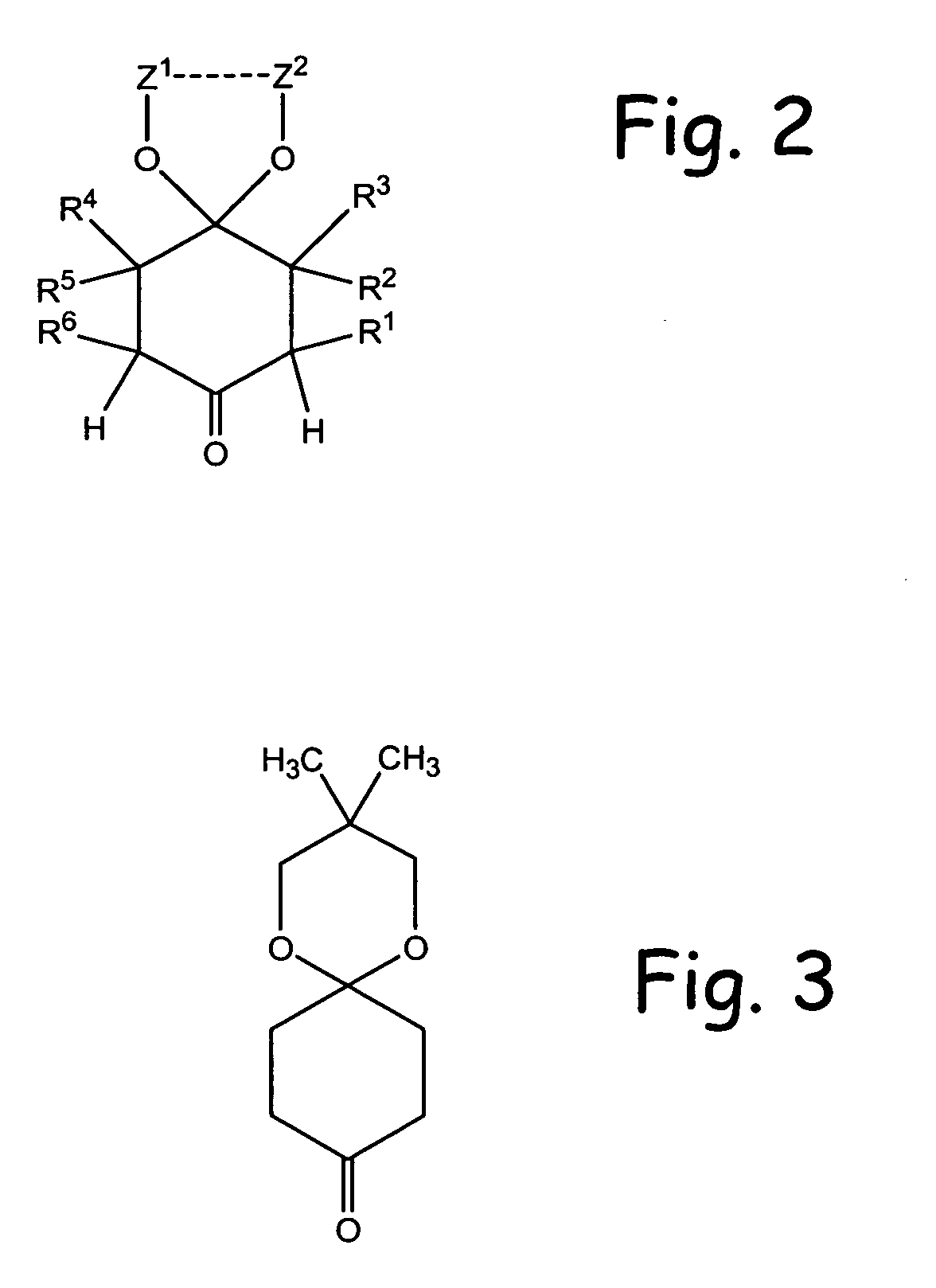
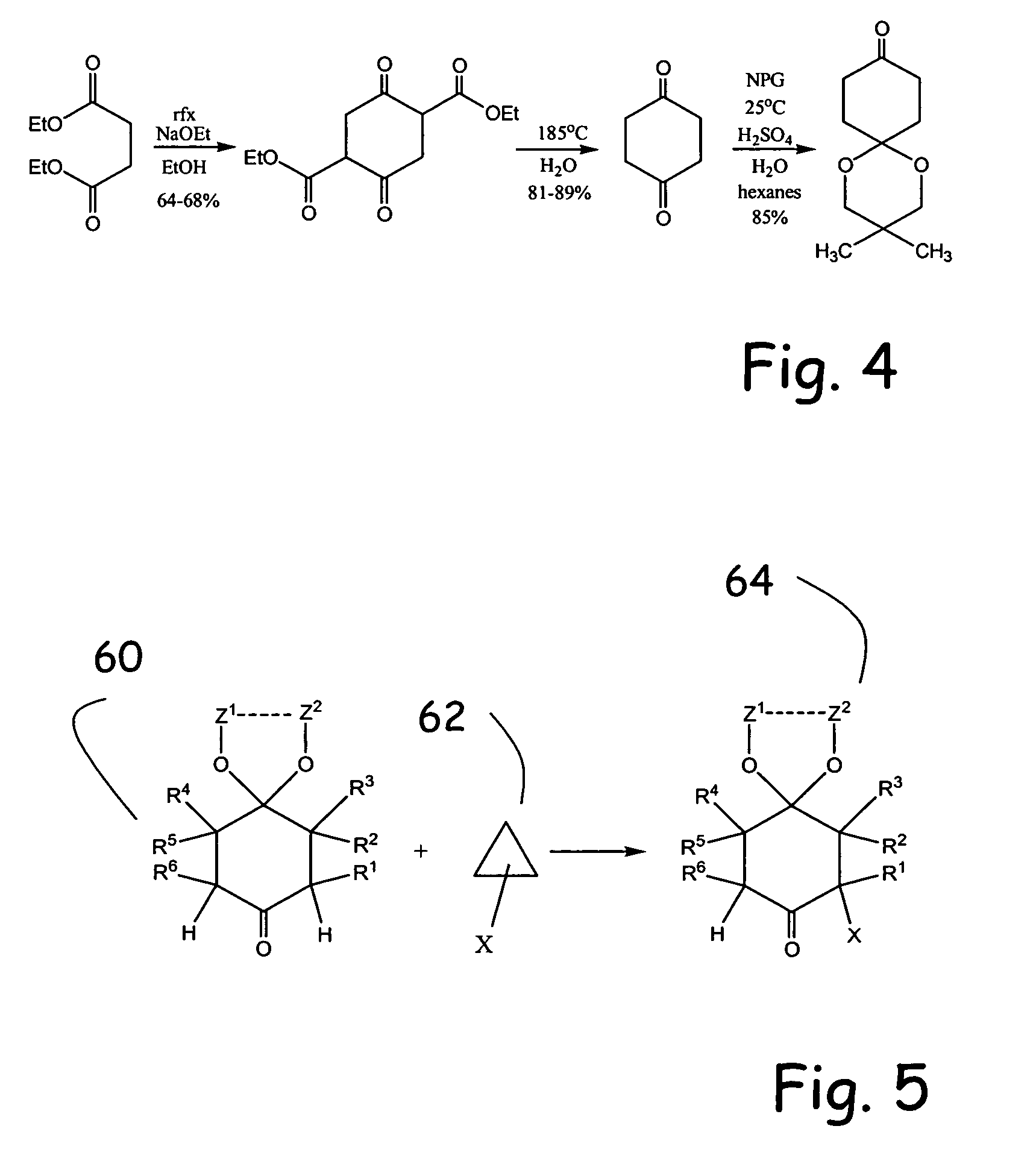
Methodologies for the alpha-monohalogenation of acid sensitive ketones, especially cyclic, acid-sensitive, ketalized ketones. As one approach, the ketone is reacted with a halogen donor compound, e.g., N-chlorosuccinimide, in anhydrous, highly polar organic reagents such as dimethylformamide (DMF). As another monohalogenation approach, it has been observed that organic salts generated from amines and carboxylic acids catalyze the monohalogenation of ketalized ketone in reagents comprising alcohol solvent (methanol, ethanol, isopropanol, etc.). The monohalogenation is fast even at −5° C. The salt can be rapidly formed in situ from ingredients including amines and / or carboxylic acids without undue degradation of the acid sensitive ketal. Aryl ketones are monooxygenated using iodosylbenzene. This methodology is applied to monohalogenation of an acid sensitive monoketal ketone. The ability to prepare monohalogenated, acid sensitive ketones facilitates syntheses using halogenated, acid sensitive ketones. As just one example, facile synthesis of halogenated, acid sensitive ketones provides a new approach to synthesize the S-ketal-acid S-MBA (S-methylbenzylamine) salt useful as an intermediate in the manufacture of a glucokinase activator. As an overview of this scheme, a monohalogenated, cyclic, ketalized ketone is prepared using monohalogenation methodologies of the present invention. The halogenated compound is then subjected to a Favorskii rearrangement under conditions to provide the racemic acid counterpart of the desired chiral salt. The desired chiral salt is readily recovered in enantiomerically pure form from the racemic mixture.
Owner:HARRINGTON PETER J +2
2-aminopyridine analogs as glucokinase activators
Provided are compounds of formula I that are useful in the treatment and / or prevention of diseases mediated by deficient levels of glucokinase activity, such as diabetes meilitus. Also provided are methods of treating or preventing diseases and disorders characterized by underactivity of glucokinase or which can be treated by activating glucokinase.
Owner:ARRAY BIOPHARMA
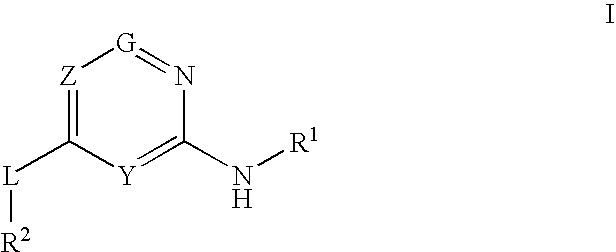
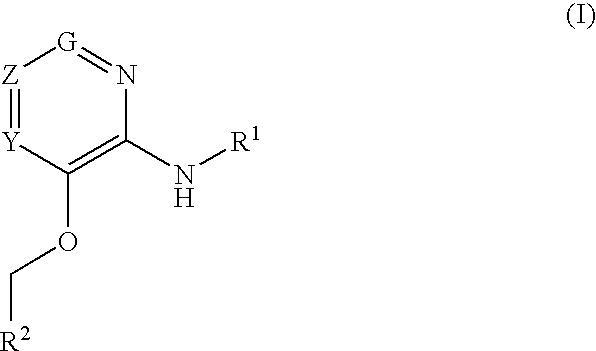
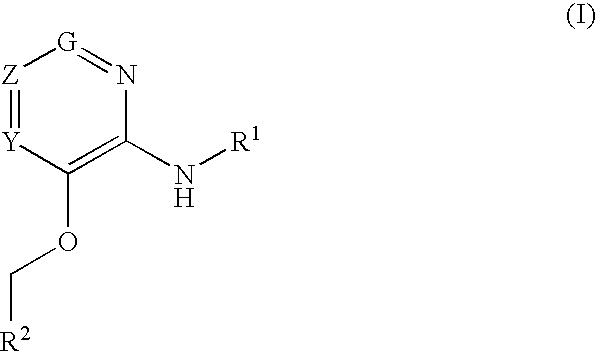
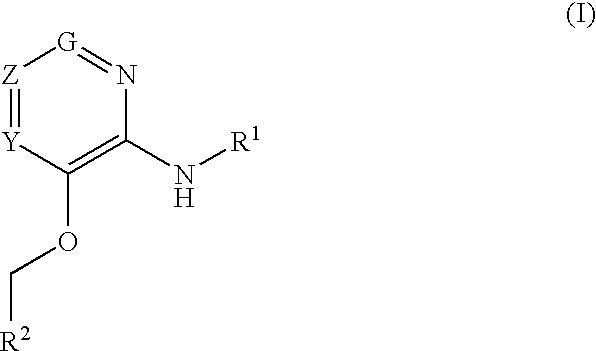
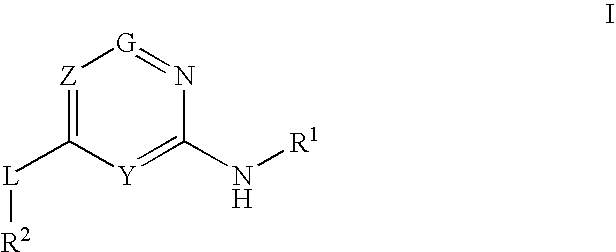
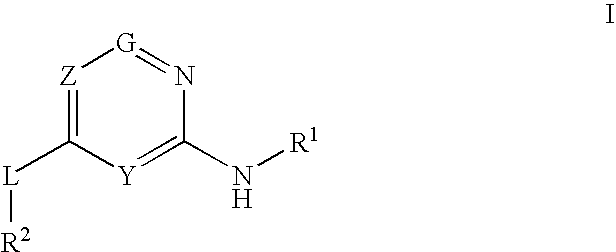
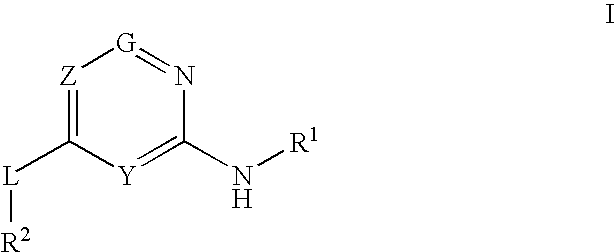
Glucokinase activators
Provided are compounds of formula Iwherein R2, L, Z, Y, G and R1 are as defined herein, that are useful in the treatment and / or prevention of diseases or disorders mediated by deficient levels of glucokinase activity or which can be treated by activating glucokinase including, but not limited to, diabetes mellitus, impaired glucose tolerance, IFG (impaired fasting glucose) and IFG (impaired fasting glycemia), as well as other diseases and disorders such as those discussed herein.
Owner:ARRAY BIOPHARMA
Glucokinase Activator Compositions for the Treatment of Diabetes
ActiveUS20140066372A1Improve glucose toleranceIncrease secretionBiocidePeptide/protein ingredientsSitagliptinAcetic acid
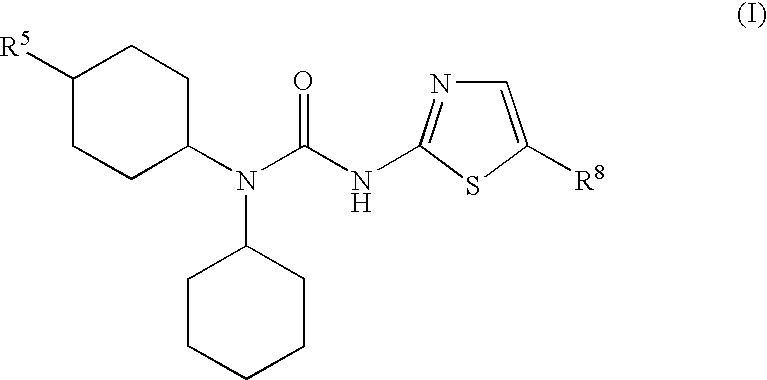
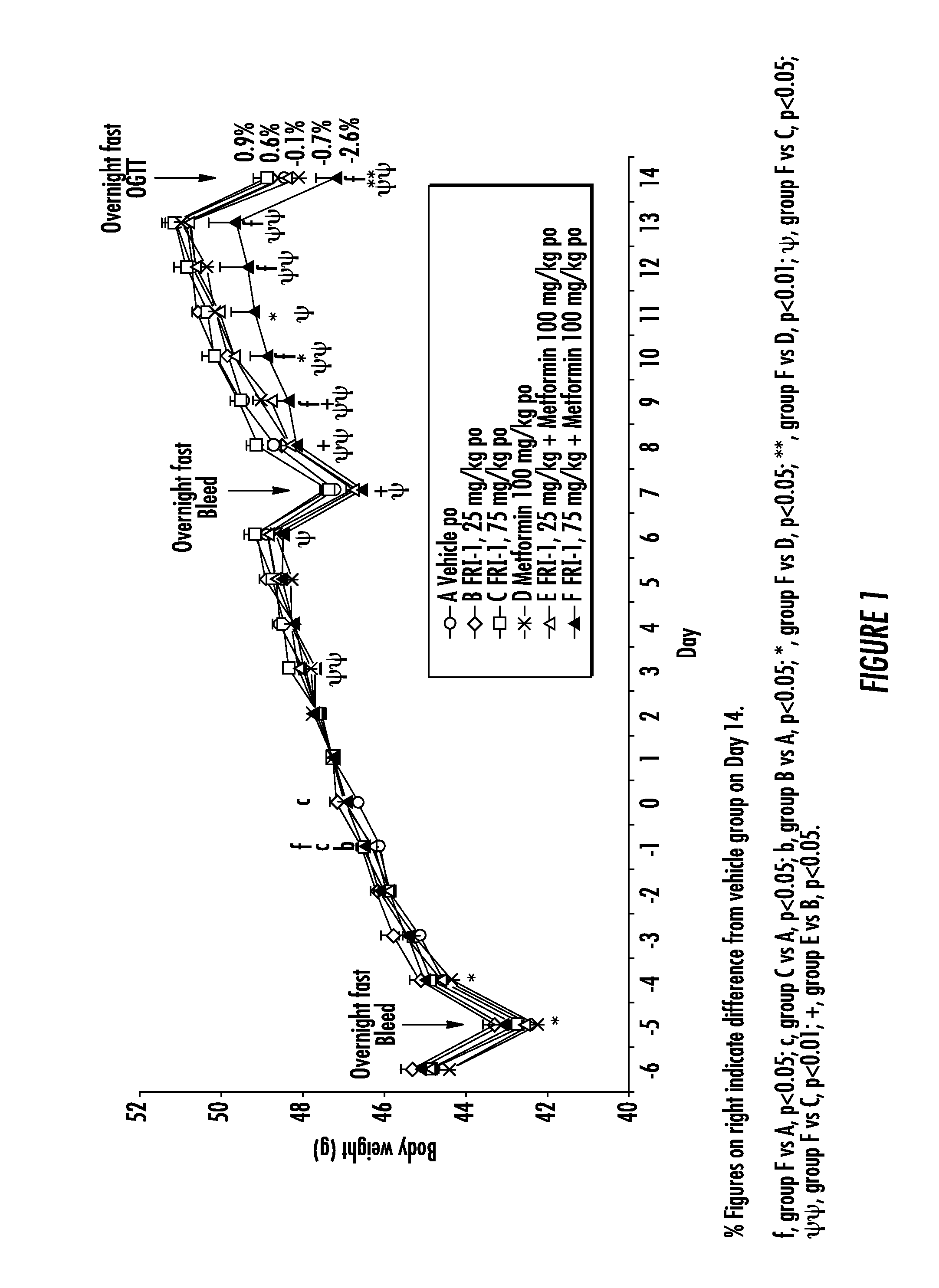
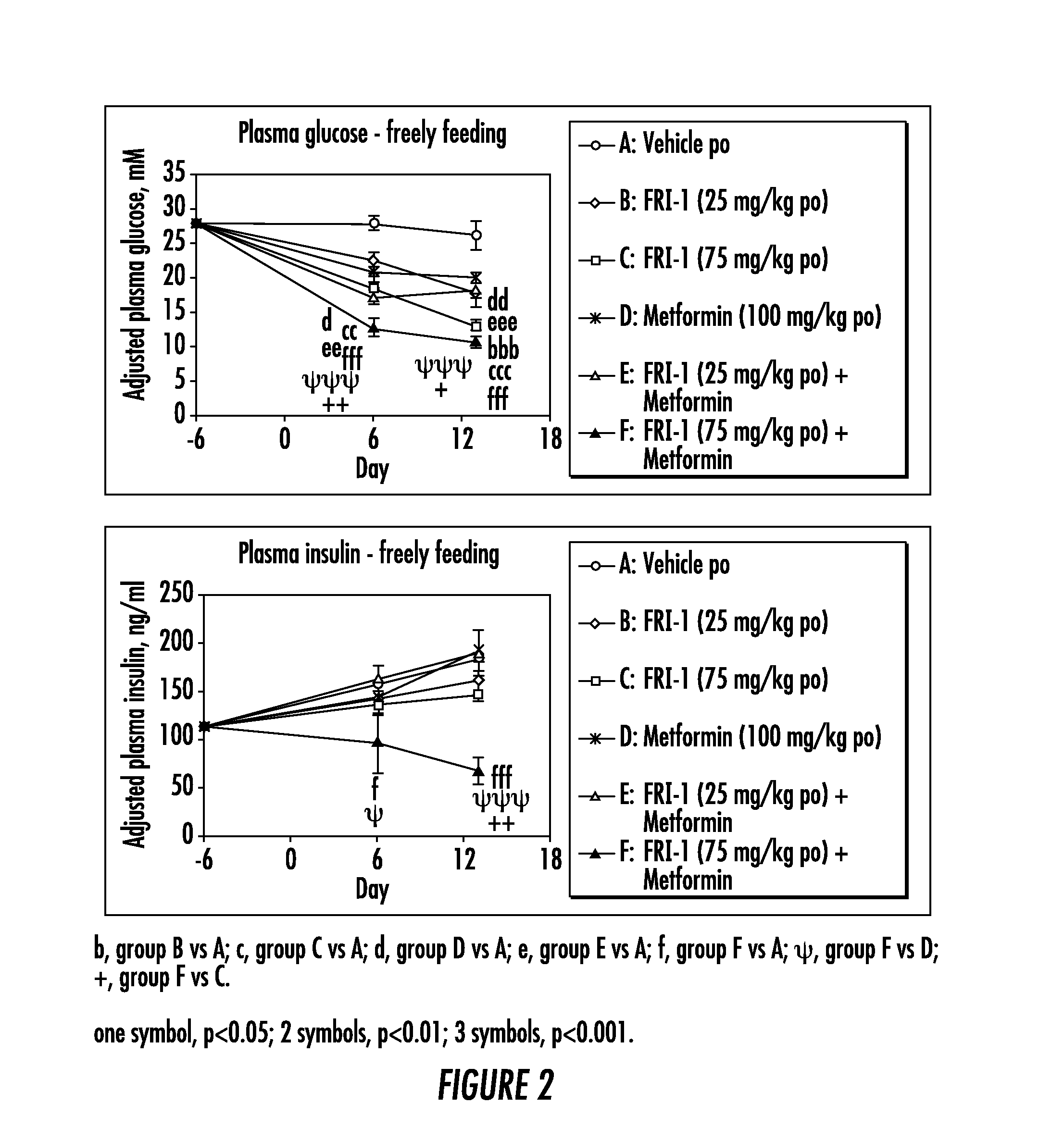
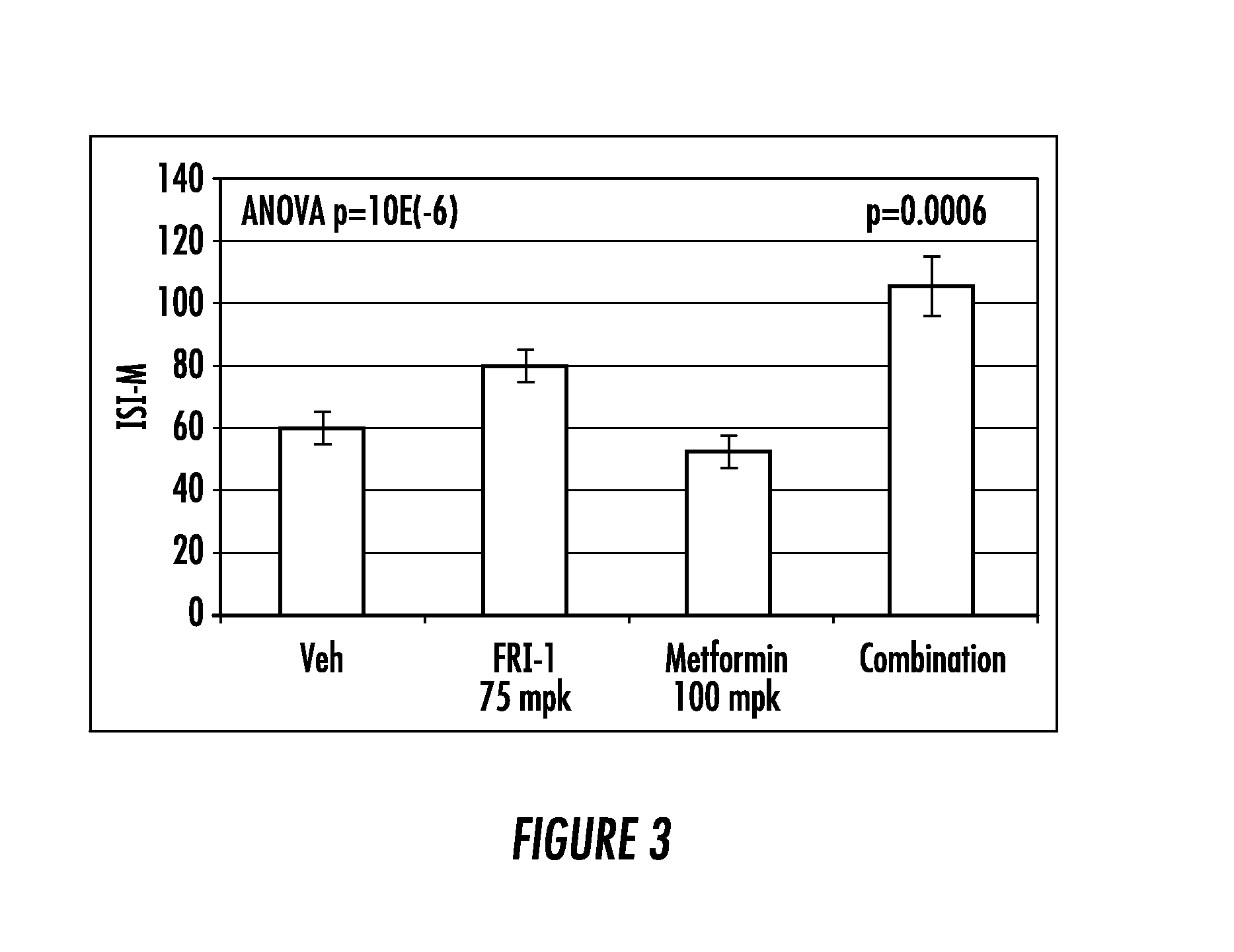
The present invention relates to pharmaceutical compositions comprising {2-[3-cyclohexyl-3-(trans-4-propoxy-cyclohexyl)-ureido]-thiazol-5-ylsulfanyl}-acetic acid (FRI-1) in combination with an anti-diabetic drug selected from the group consisting of metformin, sitagliptin or exenatide. The present invention also relates to the use of the pharmaceutical compositions in restoring insulin sensitivity and treating type II diabetes, including reducing body weight in subjects undergoing type II diabetes treatment.
Owner:VTV THERAPEUTICS LLC
Utilization of the function of rare sugar as promoter for the migration of glucokinase from nucleus to cytoplasm
Screening for a glucokinase-activating substance among rare sugars and providing a composition for treating disordered conditions in association with glucokinase activity, the composition containing the glucokinase-activating substance as the active ingredient. A promoting agent of glucokinase transfer from nucleus to cytoplasm, the promoting agent containing D-psicose and / or D-tagatose as the active ingredient, or a composition for preventing the onset of disordered conditions in association with glucokinase activity or for therapeutically treating the disordered conditions, which is in a form selected from a group consisting of food additives, food materials, drinks and foods, health drinks and foods, pharmaceutical product and feeds in blend with D-psicose and / or D-tagatose as the active ingredient for use in preventing the onset of disordered conditions in association with glucokinase activity or for therapeutically treating the disordered conditions. The disordered conditions in association with glucokinase activity are selected from impaired glucose tolerance, type 2 diabetes mellitus, insulin resistance, abnormal lipidemia, the metabolic syndrome and obesity. The composition is in a pharmaceutical form, and contains D-psicose and / or D-tagatose together with one or more pharmaceutically acceptable carriers.
Owner:KAGAWA UNIVERSITY +1
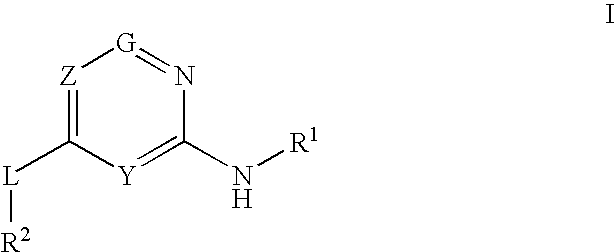
Glucokinase activators
Provided are compounds of Formula I wherein R1, R2, Y, Z and G are as defined herein, that are useful in the treatment and / or prevention of diseases mediated by deficient levels of glucokinase activity, such as diabetes mellitus. Also provided are methods of treating or preventing diseases and disorders characterized by underactivity of glucokinase or which can be treated by activating glucokinase.
Owner:ARRAY BIOPHARMA
Glucokinase activators
Provided are compounds of formula Iwherein R2, L, Z, Y, G and R1 are as defined herein, that are useful in the treatment and / or prevention of diseases or disorders mediated by deficient levels of glucokinase activity or which can be treated by activating glucokinase including, but not limited to, diabetes mellitus, impaired glucose tolerance, IFG (impaired fasting glucose) and IFG (impaired fasting glycemia), as well as other diseases and disorders such as those discussed herein.
Owner:ARRAY BIOPHARMA INC
Methods of screening for a candidate modulator of glucokinase
The present invention relates to providing novel therapeutics for treating diabetes other glycemic disorders. Such therapeutics involve the signaling pathways that contribute to regulation of glucose-stimulated insulin secretion. Of particular interest are modulators of a key component in the glucokinase pathway. Thus, the present provides methods of screening for modulators of glucokinase activity, expression, translocation, conformation, nitrosylation and interaction with other molecules as useful target for pharmacological manipulation in the treatment of diabetes and other glycemic disorders.
Owner:VANDERBILT UNIV
Oral preparation of glucokinase activator and preparation method therefor
ActiveUS20190328713A1Ensuring efficacyEnsure safetyPowder deliveryOrganic active ingredientsAcute hyperglycaemiaIGT - Impaired glucose tolerance
Disclosed is a solid dispersion and a preparation method therefor. The solid dispersion contains a glucokinase activator, an isotopic label thereof, or a medicinal salt thereof and a polymer support. Further disclosed is a solid dispersion composition containing the solid dispersion and an excipient. Also disclosed is an oral preparation of the glucokinase activator, containing the solid dispersion or the solid dispersion composition. Also disclosed is a tablet and a capsule of the glucokinase activator and a preparation method therefor. In addition, also disclosed is the uses of the solid dispersion, the solid dispersion composition and the oral preparations comprising the tablet and the capsule, which can be used for treating and / or preventing selected diseases or medical conditions and especially one or more diseases selected from type I diabetes mellitus, type II diabetes mellitus, impaired glucose tolerance, impaired fasting glucose and hyperglycemia.
Owner:HUA MEDICINE (SHANGHAI) LIMITED
Oral preparation of glucokinase activator and preparation method therefor
ActiveUS11266630B2Ensuring efficacy and safety of drugQuick releaseOrganic active ingredientsPowder deliveryDiseaseCaplet Dosage Form
Owner:HUA MEDICINE (SHANGHAI) LIMITED
2-aminopyridine analogs as glucokinase activators
Provided are compounds of formula I that are useful in the treatment and / or prevention of diseases mediated by deficient levels of glucokinase activity, such as diabetes meilitus. Also provided are methods of treating or preventing diseases and disorders characterized by underactivity of glucokinase or which can be treated by activating glucokinase.
Owner:ARRAY BIOPHARMA INC
Ureido-thiazole glucokinase activators
Owner:NOVO NORDISK AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com