Process for preparing N-phenyl-2-pyrimidyl amine derivative
An amine derivative and phenyl technology, which is applied in the field of preparation of N-phenyl-2-pyrimidineamine derivatives, can solve the problems of high price, long reaction steps, harsh reaction conditions and the like, and achieve great social and economic benefits , The effect of scientific synthesis route and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
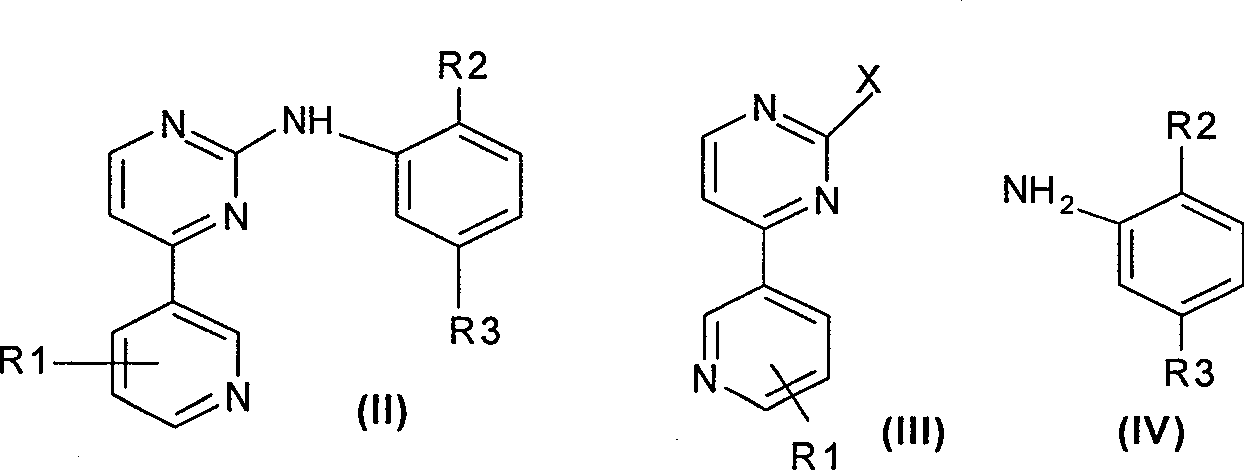
[0030] Example 1: 2-Chloro-4-(3-pyridyl)pyrimidine
[0031] 2.25 g of 3-bromopyridine was dissolved in 5 mL of anhydrous ether. Under the protection of nitrogen, 3 mL of 1.6M n-butyllithium was added dropwise at a low temperature of -40°C, and stirring was continued for 0.5 h after the dropping. After adding 3.3 g of zinc bromide in 10 mL of anhydrous ether at one time, the reaction was kept and stirred for 1 hour. Warm to room temperature, add a solution of 1.49 g of 2,4-dichloropyrimidine in 5 mL of anhydrous tetrahydrofuran and a catalytic amount of tetrakis(triphenylphosphorus) palladium, and react under reflux for 18 hours. After the reaction is completed, first extract with ethyl acetate, add dilute hydrochloric acid to the extract, and then adjust the pH to 10 with sodium hydroxide to precipitate a flocculent solid. After collection and purification, 1.65g of 2-chloro-4-(3-pyridyl)pyrimidine, mp142-143℃, 1 H-NMR(CDCl 3 ): 748 (1H, m, 5'-H), 7.71 (1H, d, 5-H), 8.45 (1H, m, 4...
Embodiment 2
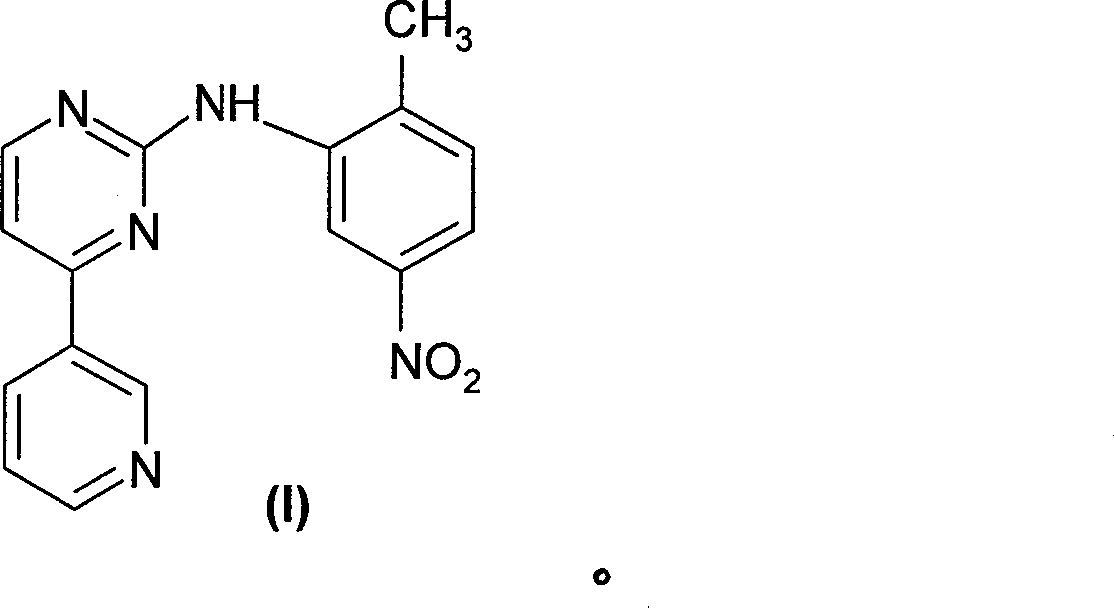
[0032] Example 2: N-(2-Methyl-4-nitrophenyl)yl-4-(3-pyridyl)-pyrimidin-2-amine
[0033] The above-mentioned 2-chloro-4-(3-pyridyl)pyrimidine 1.91g, p-nitro-o-methylaniline 1.6g, and methanesulfonic acid 0.6g were refluxed and reacted in 10 mL of anhydrous dioxane for 6 hours. After the reaction is complete, the solvent is recovered, a large amount of cold water is added to the residue, and the residue is basified with sodium bicarbonate. Collect the precipitated solid and dry it to give 2.5g, mp 195-196°C. 13 C-NMR: 164.1, 156.2, 155.5, 149.4, 148.8, 148.6, 139.7, 135.4, 132.3, 132.1, 130.2, 121.9, 121.8, 118.5, 108.9, 18.2.
Embodiment 3
[0034] Example 3: N-phenyl-4-(3-pyridyl)-pyrimidin-2-amine
[0035] Using a method similar to Example 2, 1.91 g of 2-chloro-4-(3-pyridyl)pyrimidine, 1.0 g of aniline and 0.6 g of p-toluenesulfonic acid were refluxed in 10 mL of anhydrous dioxane for 6 hours to obtain the target product , Mp.147-148℃. 13 C-NMR: 164.1, 155.5, 154.4, 151.2, 149.3, 148.8, 135.5, 132.3, 130.2, 129.3, 129.3, 122.1, 113.5, 113.5, 108.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com