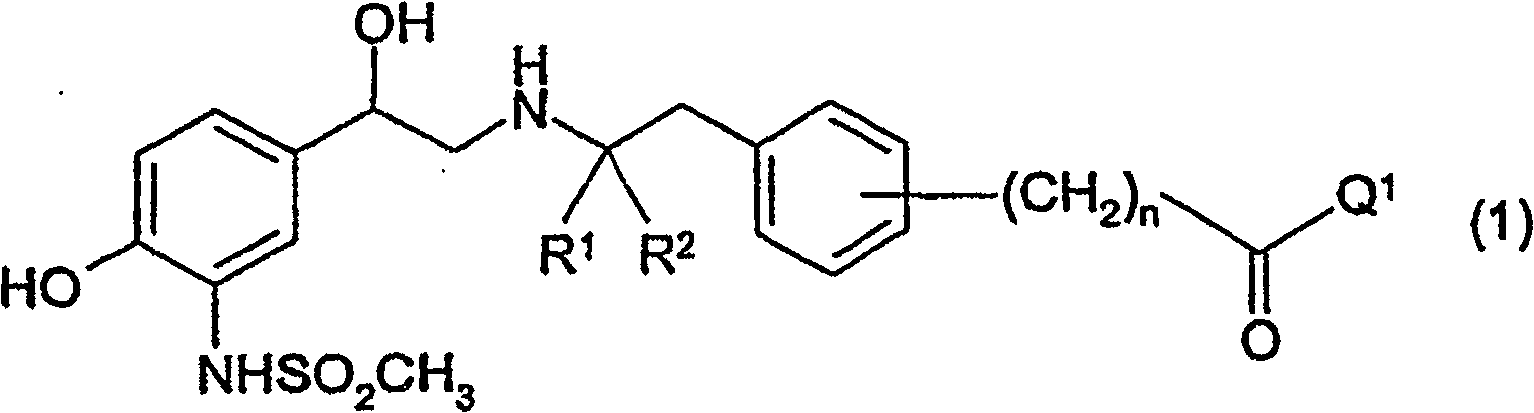
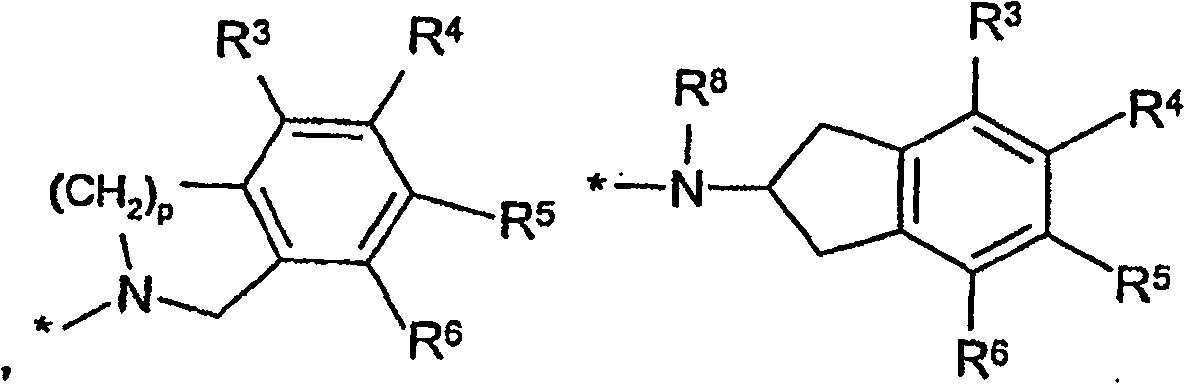
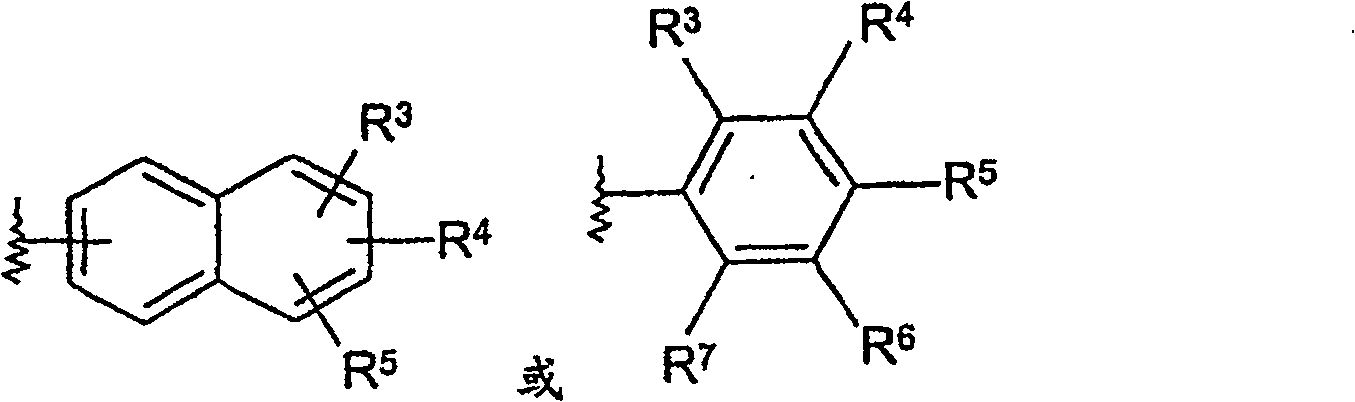
Sulfonamide derivatives for the treatment of diseases
A technology of acetamide and methylsulfonyl, applied in the field of β2 agonists, to achieve excellent efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-38
[0725] The appropriate protected alcohol (0.075 mmol) was dissolved in ethanol (4 mL) and the solution was treated with ammonium fluoride (16 mg, 0.43 mmol) in water (300 μL). The reaction mixture was then stirred at 50°C for 18 hours and allowed to cool to room temperature. If a solid product precipitated, the reaction mixture was filtered and washed with methanol:water (2 mL, 1:1 by volume) to yield the title compound. If no product precipitated, the reaction mixture was concentrated in vacuo and purified by column chromatography on silica gel eluting with dichloromethane:methanol:0.88 ammonia 98:2:0 to 95:5:0.5 to 90:10:1 residue to give the title product.
Embodiment 1
[0728] Example 1: 2-(3-{2-[((2R)-2-hydroxyl-2-[4-hydroxyl-3-[(methylsulfonyl)amino]phenyl}ethyl)amino]-2 -Methylpropyl}phenyl)-N-(4-hydroxy-3-methoxybenzyl)acetamide
[0729]
[0730] Preparation 18 (0.075 mmol) was dissolved in ethanol (4 mL) and the solution was treated with a solution of ammonium fluoride (16 mg, 0.43 mmol) in water (300 μL). The reaction mixture was then stirred at 50°C for 18 hours and allowed to cool to room temperature. The reaction mixture was concentrated in vacuo and the residue was purified by column chromatography on silica gel eluting with dichloromethane:methanol:0.88 ammonia 98:2:0 to 95:5:0.5 to 90:10:1 to give the title product of colorless solid.
[0731] 1 HNMR (CD 3 OD, 400MHz) δ: 1.04(s, 3H), 1.06(s, 3H), 2.68-2.90(m, 7H), 3.53(s, 2H), 3.74(s, 3H), 4.23(m, 2H), 4.62 (m, 1H), 6.67 (m, 2H), 6.77 (m, 1H), 6.85 (d, 1H), 7.01-7.22 (m, 6H), 7.37 (m, 1H) ppm.
[0732] MS (electrospray) m / z 572[M+H] +
Embodiment 2
[0733] Example 2: N-[(4'-hydroxybiphenyl-4-yl)methyl]-2-(3-{2-[((2R)-2-hydroxyl-2-{4-hydroxyl-3- [(methylsulfonyl)amino]phenyl}ethyl)amino]-2-methylpropyl}phenyl)acetamide
[0734]
[0735] Preparation 19 (0.075 mmol) was dissolved in ethanol (4 mL) and the solution was treated with a solution of ammonium fluoride (16 mg, 0.43 mmol) in water (300 μL). The reaction mixture was then stirred at 50°C for 18 hours and allowed to cool to room temperature. The reaction mixture was filtered and the solid was washed with methanol:water (2 mL, 1:1 by volume) to give the title compound as a colorless solid.
[0736] 1 HNMR (400MHz, DMSO d6 )δ: 0.90(s, 3H), 0.92(s, 3H), 2.56(s, 2H), 2.62-2.65(m, 2H), 2.88(s, 3H), 3.43(s, 2H), 4.25(2H , d), 4.40-4.43(m, 1H), 6.80-6.82(m, 3H), 6.96-7.01(m, 2H), 7.07-7.10(m, 2H), 7.14-7.18(m, 2H), 7.23 (d, 2H), 7.42-7.48 (m, 4H), 8.47 (t, 1H).
[0737] MS (electrospray) m / z 618[M+H] +
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap