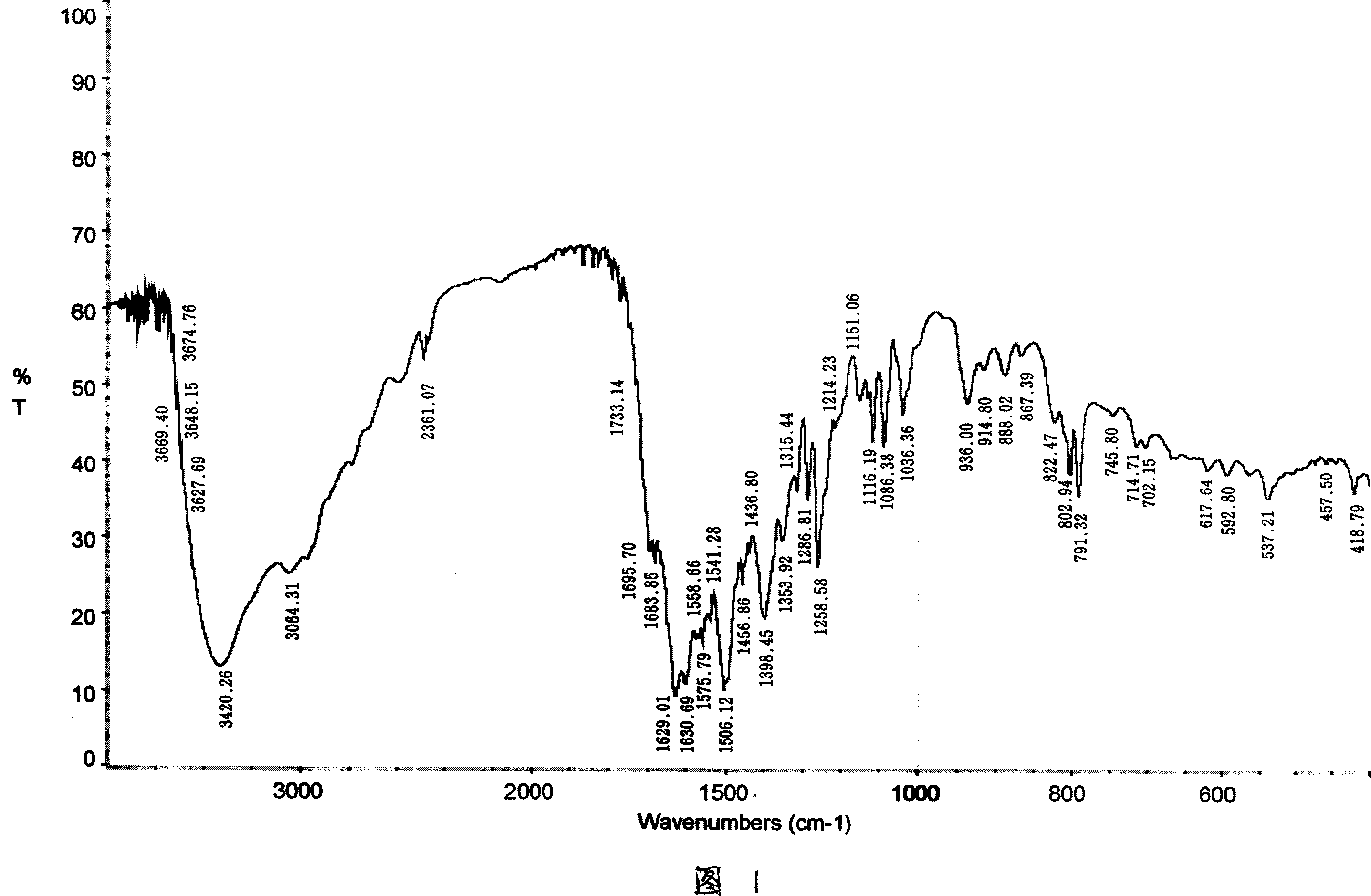
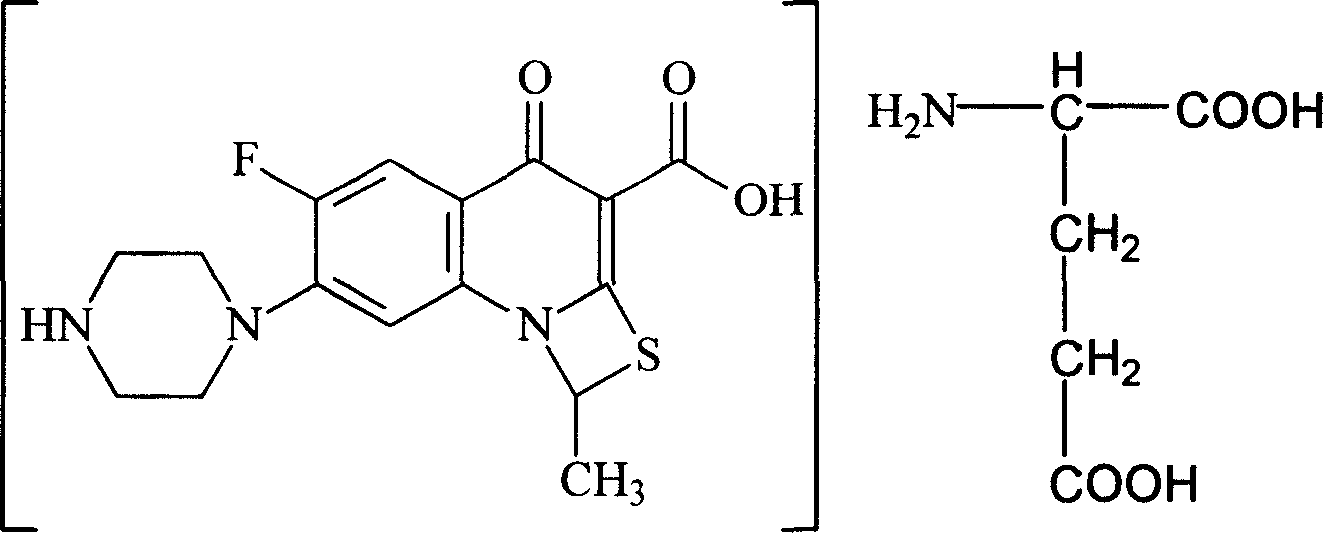
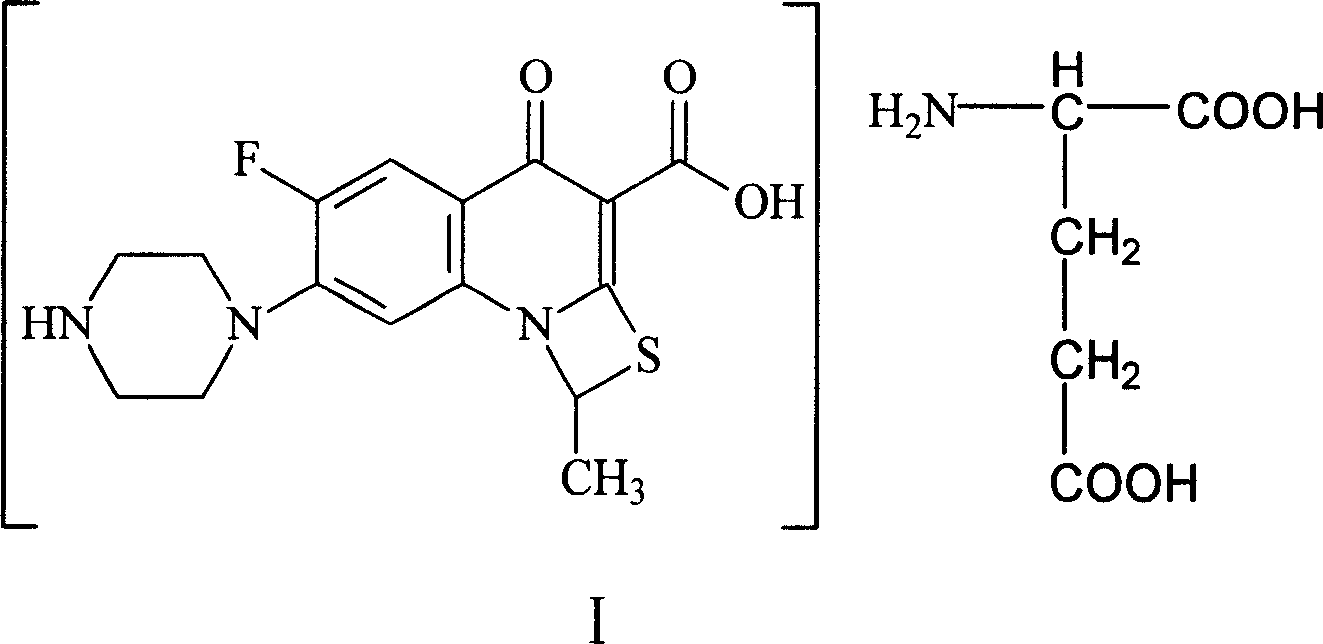
Antibiosis compound, and application
A compound, quinolone technology, applied in the field of antibacterial compounds, can solve problems such as affecting the use of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 10-15°C, add 45L water and 10L acetone to a 100L reaction tank, then add 10kg compound II, add 4.4kg glutamic acid under stirring, stir for 40 minutes, add 0.4g activated carbon for needles, stir for 15 minutes, filter to 500L In the crystallization tank; wash the reaction tank with 10L water and filter to the 500L crystallization tank. Open the crystallization tank and stir, add 400L of acetone dropwise, and the acetone is added in 4 hours, and a solid precipitates out. Keep warm at 0-5°C and stir for 4 hours, filter, wash once with 30 L of acetone, and vacuum-dry at 35-40°C. 8.6 kg of compound I were obtained.
[0053] The product elemental analysis results are as follows:
[0054] Analysis Project
Embodiment 2
[0056] 10~15°C, add 50L water and 20L isopropanol to the reaction tank, then add 10kg compound II, add 4.6kg glutamic acid under stirring, stir for 1 hour, add 0.8kg needle-use activated carbon, stir for 60 minutes, filter to remove Carbon; the filtrate is pressed into a 1000L crystallization tank in a sterile room through an ultrafiltration membrane. 15L of water washes the reaction tank and pipelines, and is also pressed into the crystallization tank. Stirring was started, and 650 L of acetone was added dropwise, and the addition of acetone was completed in 5 hours, and a solid precipitated. Keep warm at 0-5°C and stir for 5 hours, filter, wash with acetone 30L×2 twice, and vacuum-dry at 35-40°C. Obtain 8.2kg of compound I aseptic powder.
Embodiment 3
[0058] 0~5°C, add 50ml of water to a 100ml three-neck flask, then add 10g of compound II, add 4.5g of glutamic acid under stirring, stir for 30 minutes, add 0.5g of activated carbon for needles, stir for 15 minutes, filter and decarburize; 10ml Wash the three-necked flask with water and filter. Combine the filtrates into a 500ml three-necked flask, add 300ml of methanol / ethanol (1:6) dropwise under stirring, and add the mixed solution in 3 hours, and a solid precipitates out. Keep warm at 15-20°C and stir for 2 hours, filter, wash once with 20ml of absolute ethanol, and vacuum-dry at 35-40°C. 7.1 g of compound I are obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com