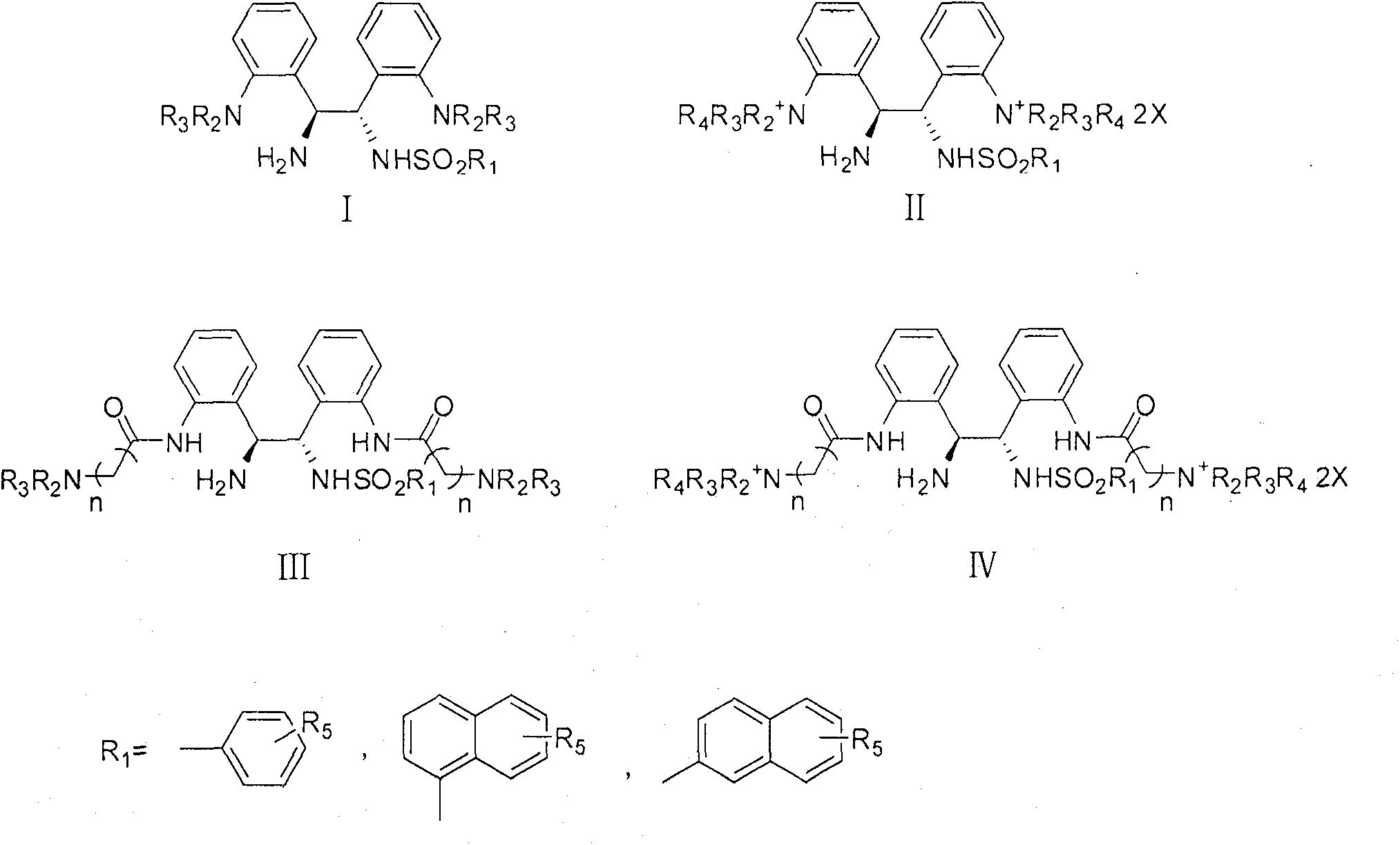
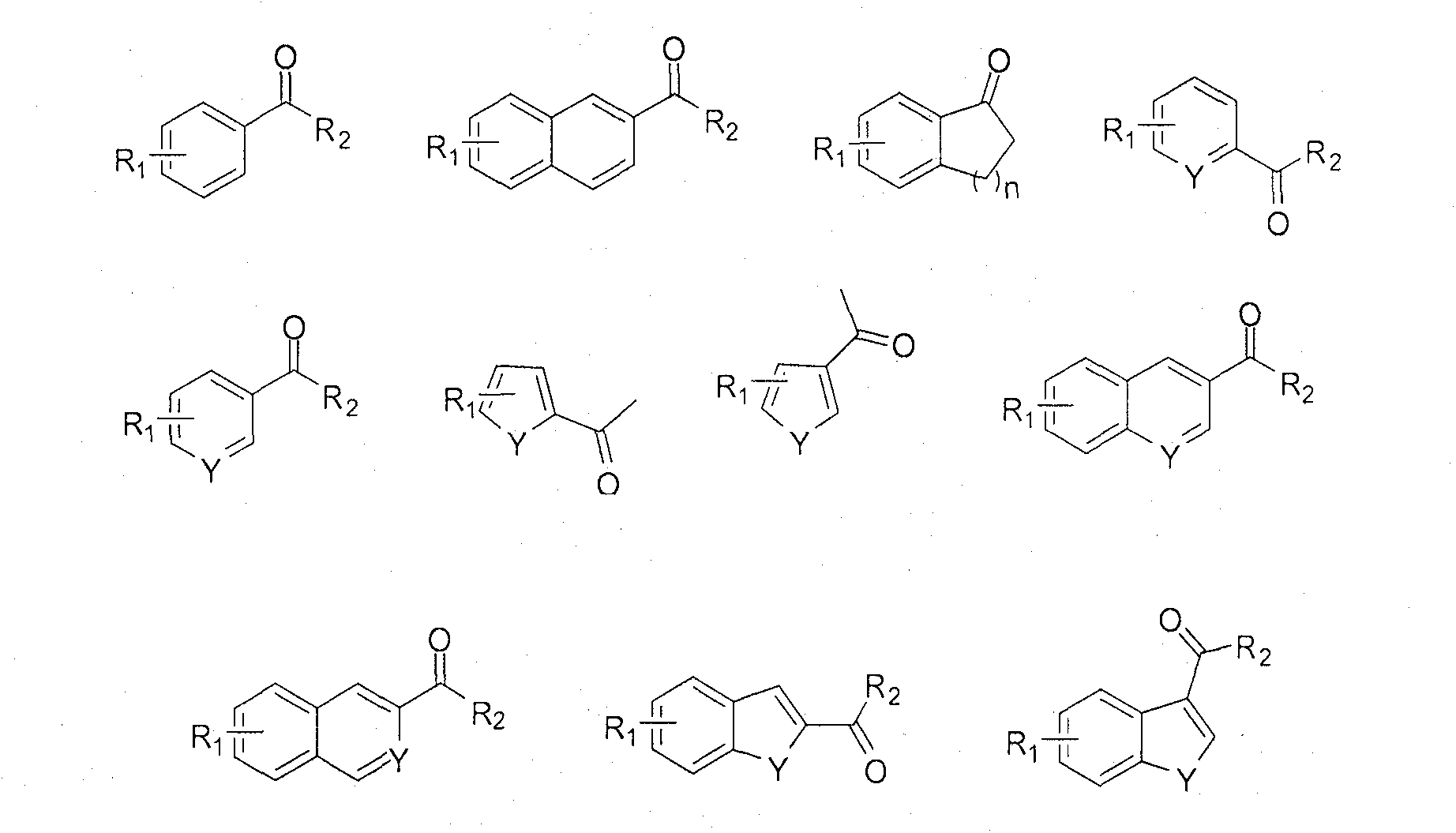
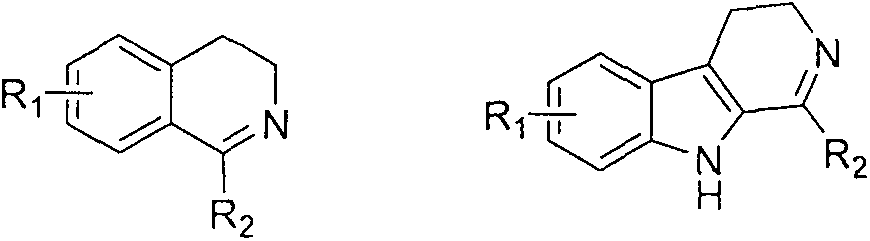Soluble chiral diamino derivative, its production and use
A chiral diamine, water-soluble technology, applied in the field of chiral diamine and its derivatives, can solve the problems of unfavorable special micro-reaction environment, stable holes, inability to form micelles or capsules, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] The asymmetric transfer hydrogenation reaction of acetophenone in the water phase that the complex of embodiment 1 ruthenium and I catalyzes
[0073] 1.2mg (0.002mmol) of [RuCl 2 (p-cymene)] 2 and 1.7mg (0.0044mmol) of water-soluble ligand N-tosylated-1,2-bis-(2'-aminophenyl)-1,2-ethylenediamine were added to 1mL of water and activated at 40°C 1 hour. At room temperature, 208mg (2.0mmol) HCOONa·2H 2 O and 48 μL (0.4 mmol) of acetophenone were added to the system and reacted for 0.5 hours. Then use CH 2 Cl 2 After extraction several times, the extract was dried with anhydrous sodium sulfate, filtered and concentrated, and the conv. and ee values were determined by GC (33% conv., 95% ee).
Embodiment 2
[0074] The asymmetric transfer hydrogenation reaction of acetophenone in the aqueous phase of the complex catalysis of embodiment two iridium and I
[0075] 1.6mg (0.002mmol) of [IrCl 2 (cp * )] 2 and 1.7mg (0.0044mmol) of water-soluble ligand N-tosylated-1,2-bis-(2'-aminophenyl)-1,2-ethylenediamine were added to 1mL of water and activated at 40°C 1 hour. At room temperature, 208mg (2.0mmol) HCOONa·2H 2 O and 48 μL (0.4 mmol) of acetophenone were added to the system and reacted for 0.5 hours. Then use CH 2 Cl 2 After extraction several times, the extract was dried with anhydrous sodium sulfate, filtered and concentrated, and the conv. and ee values were determined by GC (29% conv., 95% ee).
[0076] Asymmetric transfer hydrogenation of acetophenone in aqueous phase catalyzed by complexes of rhodium and I
Embodiment 3
[0078] 1.3mg (0.002mmol) of [RhCl 2 (cp * )] 2 and 1.7mg (0.0044mmol) of water-soluble ligand N-tosylated-1,2-bis-(2'-aminophenyl)-1,2-ethylenediamine were added to 1mL of water and activated at 40°C 1 hour. At room temperature, 41.6mg (0.4mmol) HCOONa·2H 2 O and 48 μL (0.4 mmol) of acetophenone were added to the system and reacted for 0.5 hours. Then use CH 2 Cl 2 After extraction several times, the extract was dried with anhydrous sodium sulfate, filtered and concentrated, and the conv. and ee values were determined by GC (86% conv., 97% ee).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com