Process for preparing irinotecan hydrochloride medicines
A technology for irinotecan hydrochloride and drugs, which is applied in the field of preparation of irinotecan hydrochloride drugs, can solve the problems of large solvent demand, low efficiency, and impure products, and achieve improved production efficiency, high product purity, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
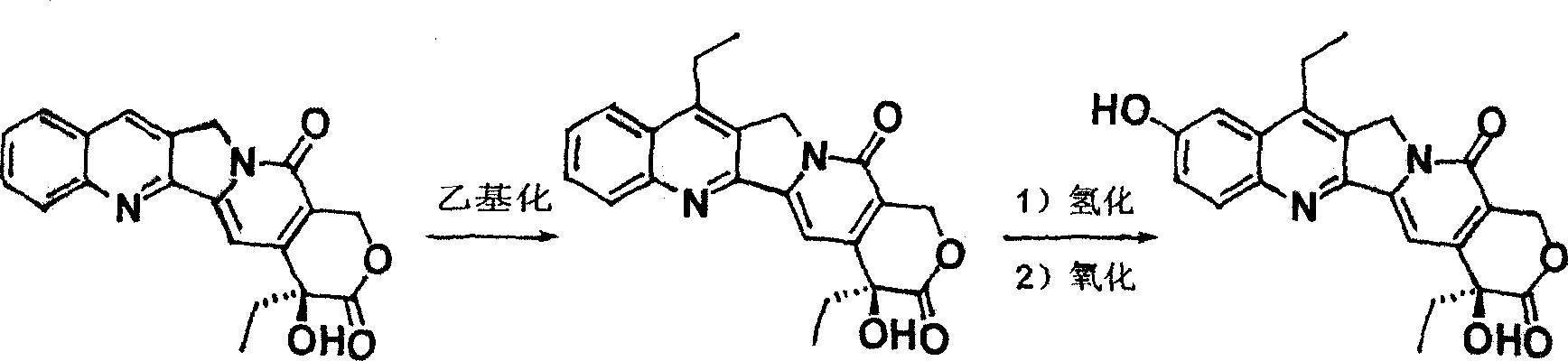
[0019] The preparation method of the present embodiment irinotecan hydrochloride medicine comprises the steps:
[0020] 1) Camptothecin ethylation reaction to obtain 7-ethylcamptothecin;
[0021] 2) Hydrogenation of 7-ethylcamptothecin to obtain hydrogenated-7-ethylcamptothecin;
[0022] 3) Oxidation of hydrogenated-7-ethylcamptothecin to obtain 7-ethyl-10-hydroxycamptothecin;
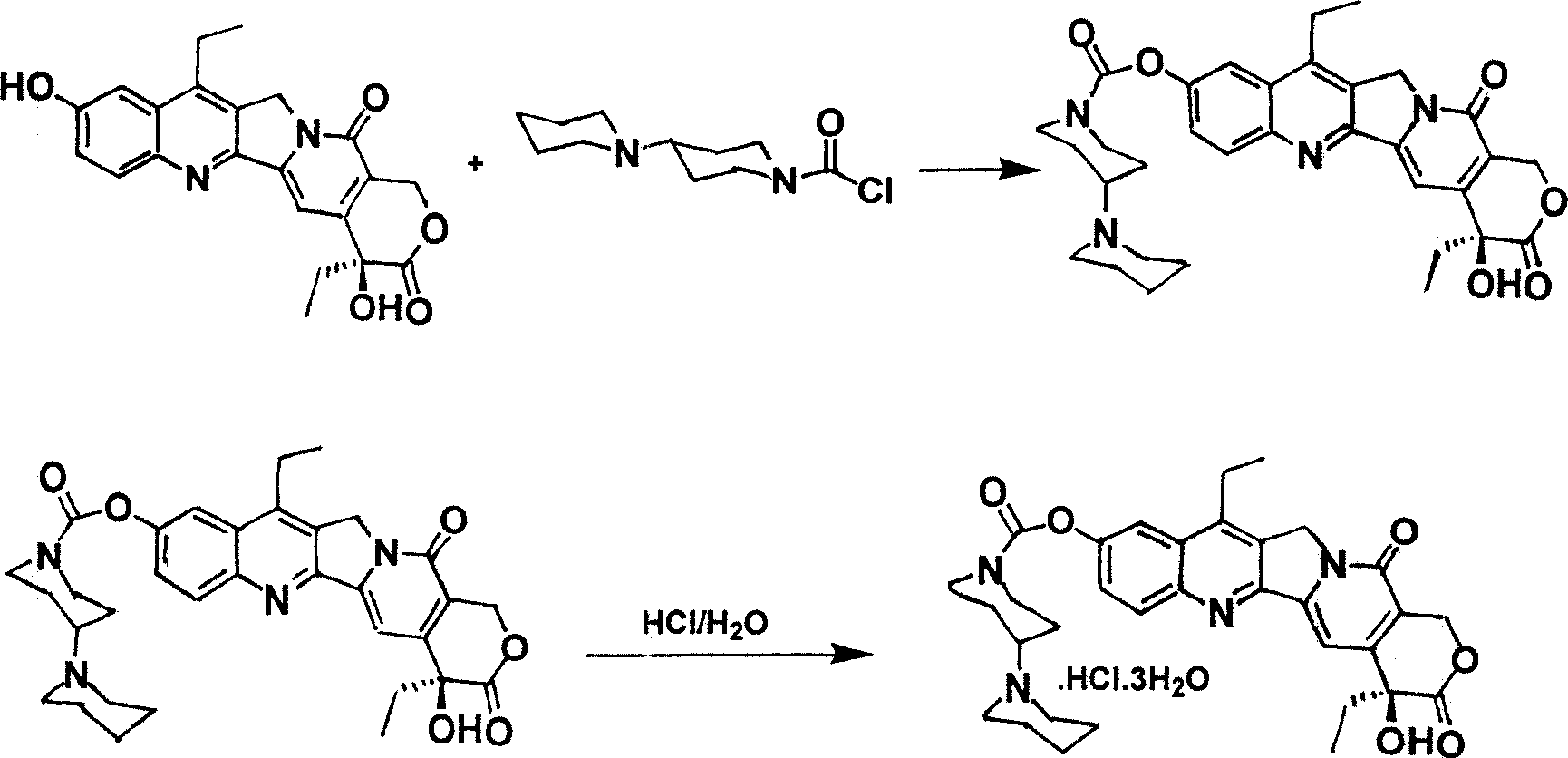
[0023] 4) Coupling reaction of 7-ethyl-10-hydroxycamptothecin and 4-piperidinylpiperidinecarbonyl chloride to prepare irinotecan hydrochloride.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap