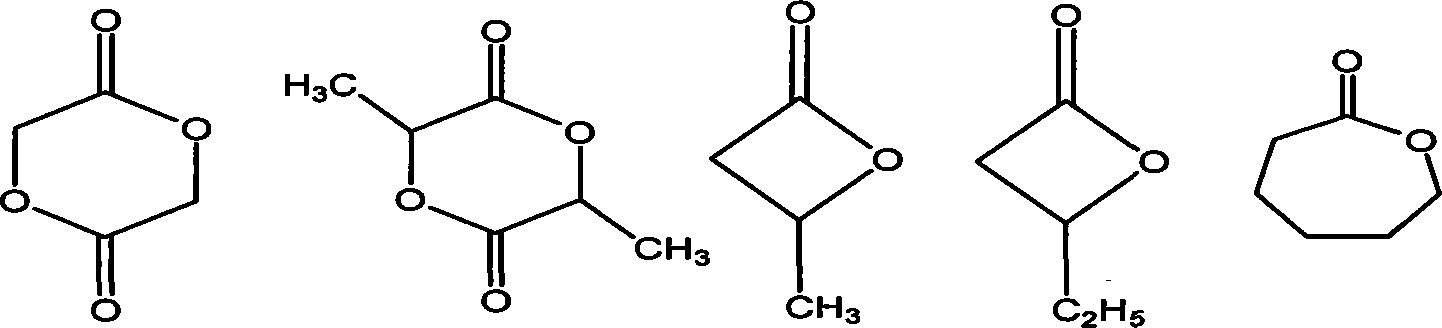
Method for catalyzing polymerization of cyclic lactone
A technology of cyclic lactones and compounds, applied in the field of catalytic chemistry, which can solve the problems of low reactivity and relatively long reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Homopolymer 1 of ε-caprolactone
[0024] All operations were carried out under the protection of anhydrous, oxygen and inert gas. In the ampoule after being washed and baked with high-purity argon, add measured amounts of toluene, ε-caprolactone, and the mixture of amine imine aluminum catalyst and benzyl alcohol in sequence, and place it in an oil bath at -20 to 250°C middle. After the reaction is completed, toluene containing a small amount of water is added to terminate the reaction and dissolve the polymer, precipitate with methanol, wash several times, and dry under vacuum at room temperature.
[0025] Polymerization adopts solution polymerization method, with toluene as solvent, adding monomer ε-caprolactone (2.0ML, 19mmol), (2-phenyliminomethyl) phenylanilinodimethylaluminum (0.19mmol) and A mixture of benzyl alcohol (0.19 mmol) and a concentration of ε-caprolactone of 3.0 mol / L was polymerized at 70°C. After reacting for 10 minutes, add toluene cont...
Embodiment 2
[0026] Example 2 Homopolymer 2 of ε-caprolactone
[0027] Polymerization adopts solution polymerization method, with toluene as solvent, adding monomer ε-caprolactone (2.0ML, 19mmol), (2-phenyliminomethyl) phenylanilinodimethylaluminum (0.19mmol) and A mixture of benzyl alcohol (0.38mmol) and a concentration of ε-caprolactone of 3.0mol / L was polymerized at 70°C. After reacting for 15 minutes, toluene containing a small amount of water was added to terminate the reaction and dissolve the polymer, precipitate with methanol, wash several times, and dry under vacuum at room temperature. Obtain polycaprolactone 1.97 grams, productive rate 91.6%, Mn is 14.6 * 10 3 , Mn determined by GPC polystyrene as a standard.
[0028] When the mol ratio of benzyl alcohol / Al was 0.01, 2.0 grams of polycaprolactone was obtained in 20 minutes after reaction, the productive rate was 93.0%, and Mn was 213×10 3 .When the mol ratio of benzyl alcohol / Al is 200, react 30 minutes to obtain polycaprolac...
Embodiment 3
[0029] Example 3 Homopolymer 3 of ε-caprolactone
[0030] Polymerization adopts solution polymerization method, with toluene as solvent, adding monomer ε-caprolactone (2.0ML, 19mmol), (2-phenyliminomethyl) phenylanilinodimethylaluminum (0.19mmol) and A mixture of benzyl alcohol (0.095mmol) and a concentration of ε-caprolactone of 3.0mol / L was polymerized at 70°C. After reacting for 1.5 minutes, add toluene containing a small amount of water to terminate the reaction and dissolve the polymer, precipitate with methanol, wash several times, and vacuum dry at room temperature. Obtain polycaprolactone 2.1 grams, productive rate 97.6%, Mn is 35.4 * 10 3 , Mn determined by GPC polystyrene as a standard.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap