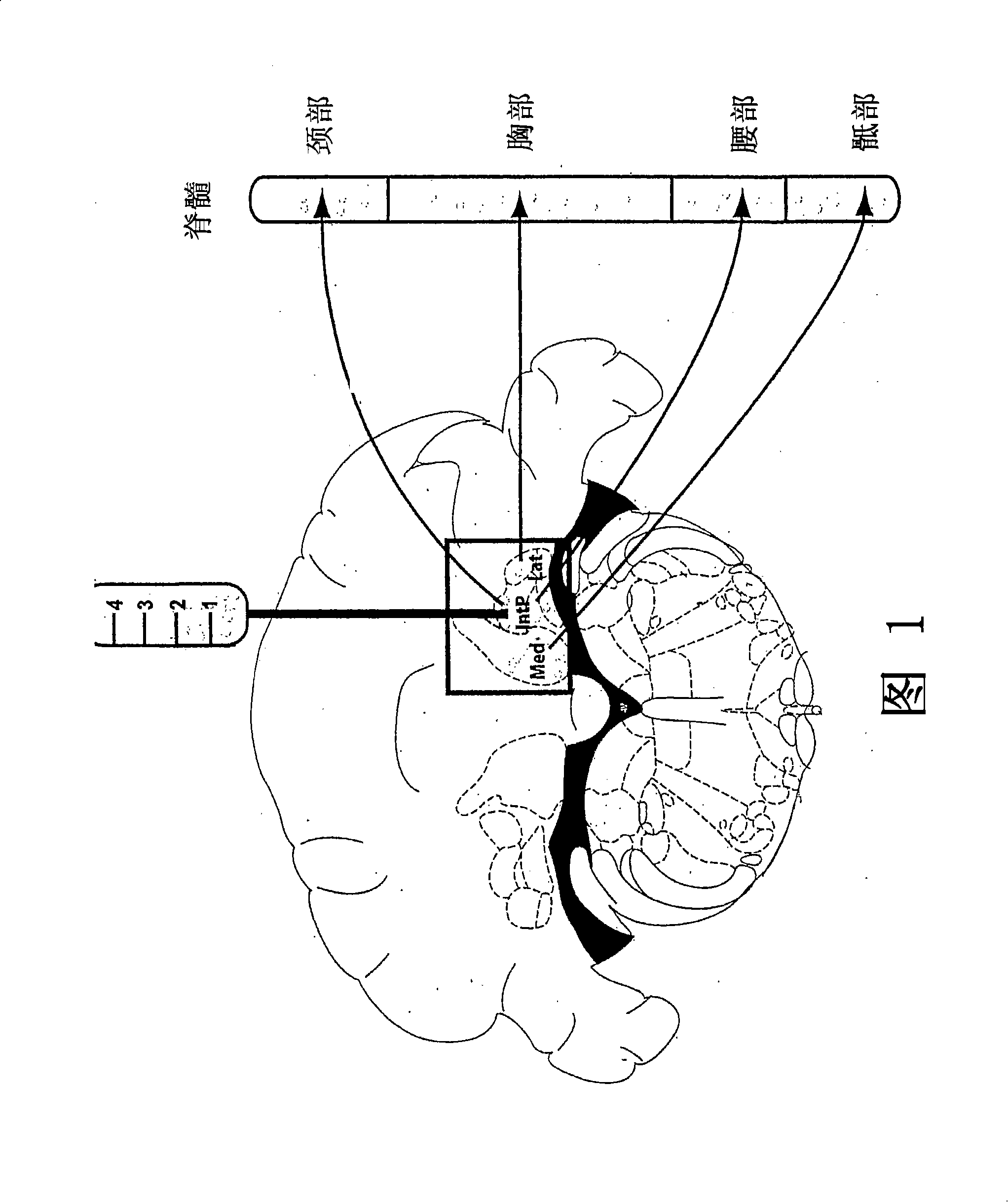
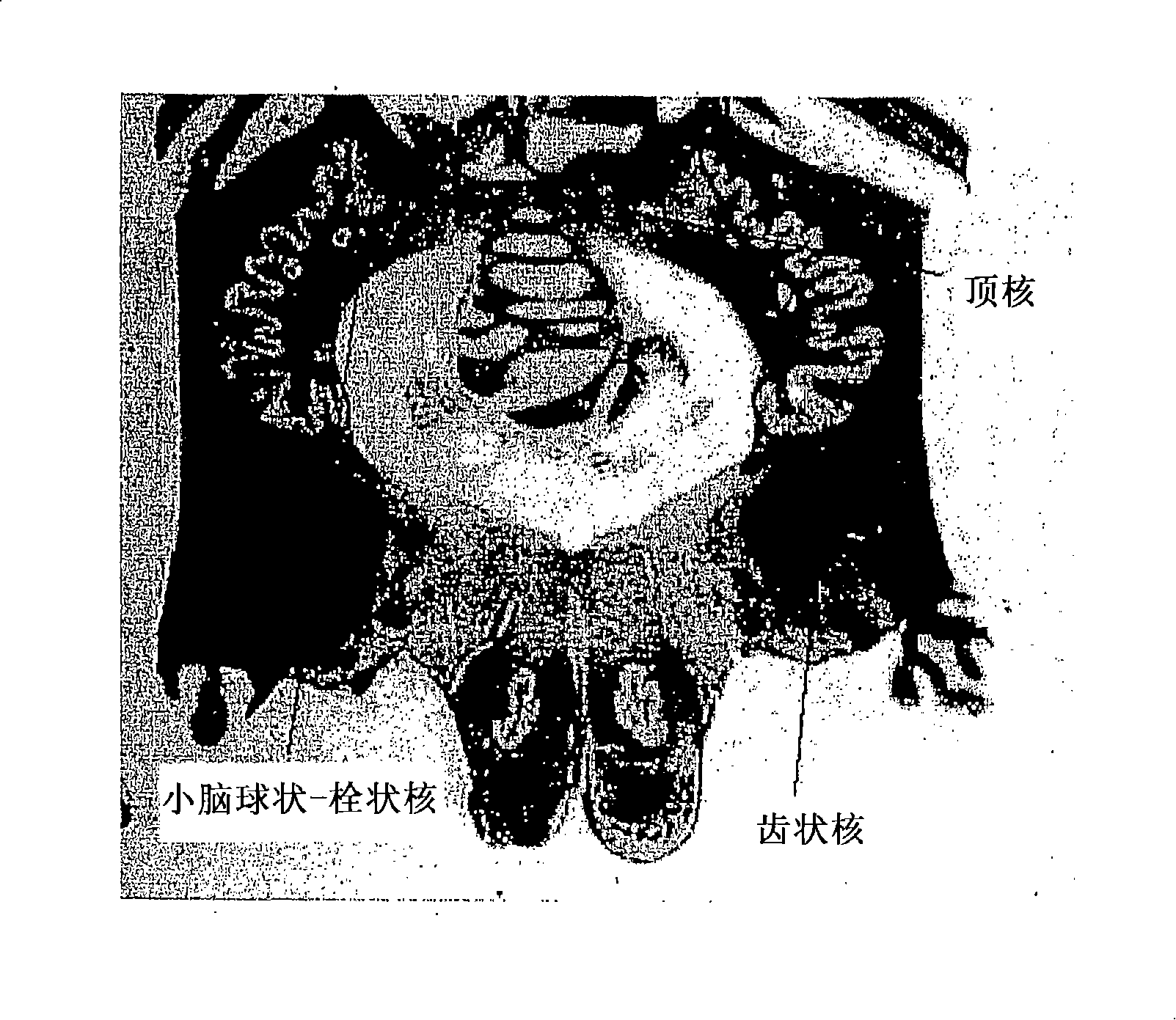
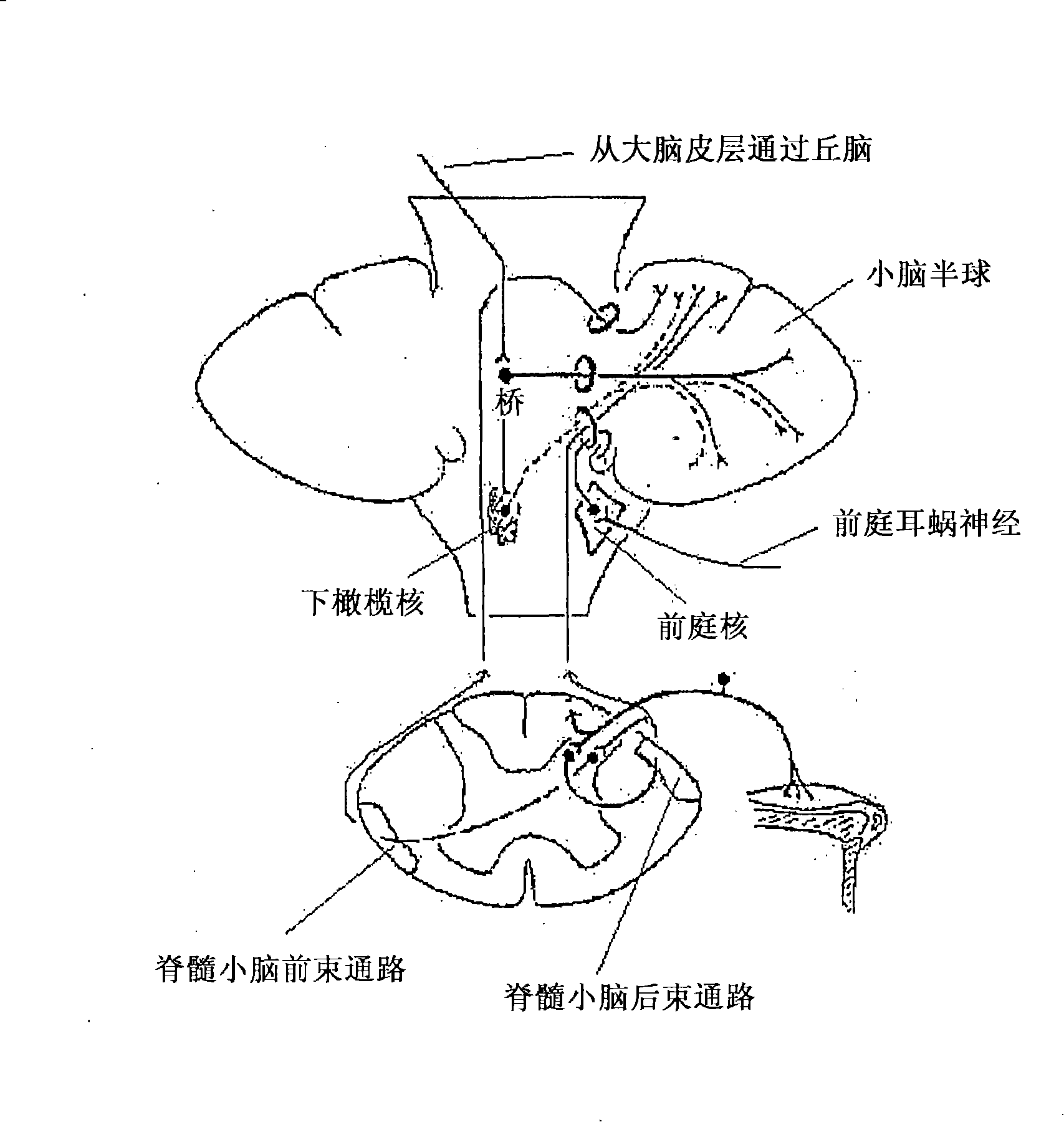
Gene therapy for spinal disorders
A spinal cord, transgenic technology, applied in the field of gene therapy for spinal cord disorders, can solve the problems of patients with loss of motor function and still existing needs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0099] [099] The following examples provide illustrative embodiments of the invention. One of ordinary skill in the art will recognize numerous modifications and variations that could be implemented without altering the spirit or scope of the invention. Such modifications and changes are included within the scope of the present invention. These examples do not limit the invention.
[0100] Example
[0101] Titration of recombinant vector
[0100] Titers of AAV vectors were measured in terms of genome copy number (genome particles per milliliter). Genomic Particle Concentration Based on Taqman of Vector DNA PCR, as previously reported (Clark et al., (1999) Hum. Gene Ther., 10:1031-1039; Veldwijk et al., (2002) Mol. Ther, 6:272-278). Briefly, purified AAV-ASM was treated with capsid protein digestion buffer (50 mM Tris-HCl pH 8.0, 1.0 mM EDTA, 0.5% SDS, 1.0 mg / ml proteinase K) at 50°C for 1 hour to release vector DNA. DNA samples are subjected to polymerase chain reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com