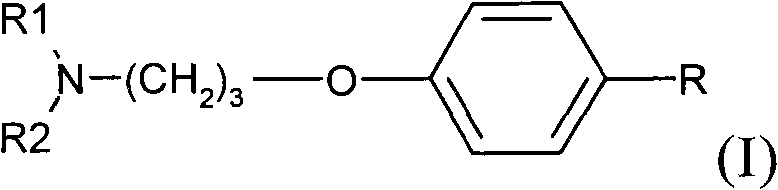
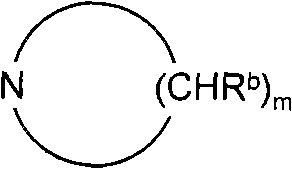
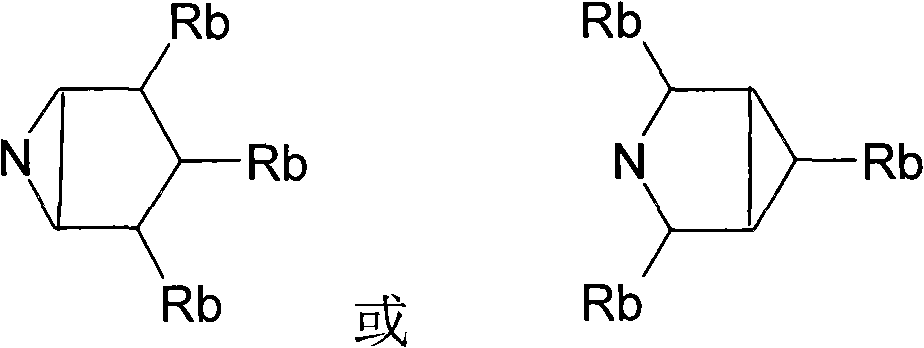
Phenoxypropylpiperidines and -pyrrolidines and their use as histamine h3-receptor ligands
An Alkyl, Alkenyl Technology Applied in the Field of New H3-Receptor Ligands
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0358]
[0359] trans-1-{3-[4-(N,N-Dimethylcarbamoyl)phenoxy]propyl}-3,5-dimethylpiperidine oxalate
[0360]
[0361] 4-[3-(cis and trans-3,5-dimethylpiperidinyl)propoxy]benzoyl chloride hydrochloride (866 mg), 2M dimethylamine in tetrahydrofuran (3.75 mL) and tetrahydrofuran ( 10 mL) was stirred at room temperature for 1 hour. The mixture was concentrated under reduced pressure and purified by chromatography using silica gel and a mixture of diethyl ether / naphtha / triethylamine (80 / 20 / 1) as eluent. Fractions containing the expected product were collected, concentrated under reduced pressure and salified with 20 mg oxalic acid to give 37 mg trans-1-{3-[4-(N,N-dimethylcarbamoyl)-phenoxy] Propyl}-3,5-dimethylpiperidine oxalate, which is a pink powder.
[0362] 1 H NMR: Base (CDCl 3 )
[0363] 7.38(d, J=8.7Hz, 2H, arm), 6.90(d, J=8.7Hz, 2H, arm), 4.05(t, J=6.0Hz, 2H, CH 2 O), 3.05(br s, 6H, 2CH 3 NCO), 2.5-1.8(m, 10H, 3CH 2 N, CH 2 , 2CH), 1.25 (dd, J=5.8Hz, J=5.8Hz...
Embodiment 2
[0381]
[0382] trans-1-{3-[4-(N,N-tetramethylenecarbamoyl)phenoxy]propyl}-3,5-dimethylpiperidine, oxalate
[0383] Following the procedure described in Example 1§A, but starting from 4-[3-(cis and trans-3,5-dimethylpiperidinyl)propoxy]benzoyl chloride hydrochloride (700 mg) and A solution of pyrrolidine (500 μL) in dichloromethane (10 mL) gave 24 mg of trans-1-{3-[4-(N,N-tetramethylenecarbamoyl)phenoxy]propyl}-3,5 -Dimethylpiperidine oxalate as a white powder.
[0384] 1 H NMR: Base (CDCl 3 )
[0385] 7.51(d, J=8.7Hz, 2H, arm), 6.90(d, J=8.7Hz, 2H, arm), 4.06(t, J=6.0Hz, 2H, CH 2 O), 3.55(m, 4H, 2CH 2 NCO), 2.5-1.8(m, 14H, 3CH 2 N,3CH 2 , 2CH), 1.25 (dd, J=5.8Hz, J=5.8Hz, 2H, CH 2 ), 0.95 (2d, J=6.8Hz, J=6.8Hz, 6H, 2CH 3 )
Embodiment 3
[0387]
[0388] 1-[3-(4-Benzoylphenyl)propoxy]piperidine oxalate
[0389]A Following the procedure described in Example 1§D, but starting with 4-(3-chloropropoxy)benzophenone (1.37 g), piperidine (1 mL), potassium carbonate (2.07 g) and catalyst Quantitative potassium iodide in N,N-dimethyl-formamide (25 mL) gave 239 mg of 1-[3-(4-benzoylphenyl) after generating the salt with oxalic acid (55 mg) in acetone (0.6 mL) ) Propoxy]piperidine oxalate, which is a white powder.
[0390] 1 H NMR: Oxalate (DMSO)
[0391] 7.73 (d, J = 8.8Hz, 2H, aroma), 7.67-7.53 (m, 5H, Ph), 7.07 (d, J = 8.8Hz, 2H, aroma), 4.15 (t, J = 6.0Hz, 2H, CH 2 O), 3.09(m, 6H, 3CH 2 N), 2.15(m, 2H, CH 2 ), 1.70 (m, 4H, 2CH 2 ), 1.50 (m, 2H, CH 2 )
[0392] B 4-(3-chloropropoxy)benzophenone can be prepared as follows:
[0393] Following the procedure described in Example 1§E, but starting with N of 4-hydroxybenzophenone (0.99 g), potassium carbonate (3.45 g) and 1-bromo-3-chloropropane (2.5 mL), A sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap