Borohydride direct oxidation fuel cell electrocatalyst
A technology of fuel cells and electrocatalysts, applied in the direction of metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, battery electrodes, etc., can solve the problems of methanol penetration, hydrogen storage, poor portability, etc., to achieve Effect of reducing decomposition and improving fuel efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Hydrothermal TiO 2 Preparation: take 20ml of titanium isopropoxide and 4.2g of glacial acetic acid, quickly add 100ml of water and stir for one hour to make it completely hydrolyzed, then add 1.5ml of 65%wt nitric acid, and bathe in water at 78°C for 75 minutes. The measured pH of the solution is about is 1.5. Continue to hydrothermally react the solution at 240° C. for 12 hours, then add 0.8 ml of 65% wt nitric acid, and sonicate for one hour at 200 W, at which point the measured pH is 3-3.5.
[0035] Preparation of electro-oxidation catalyst:
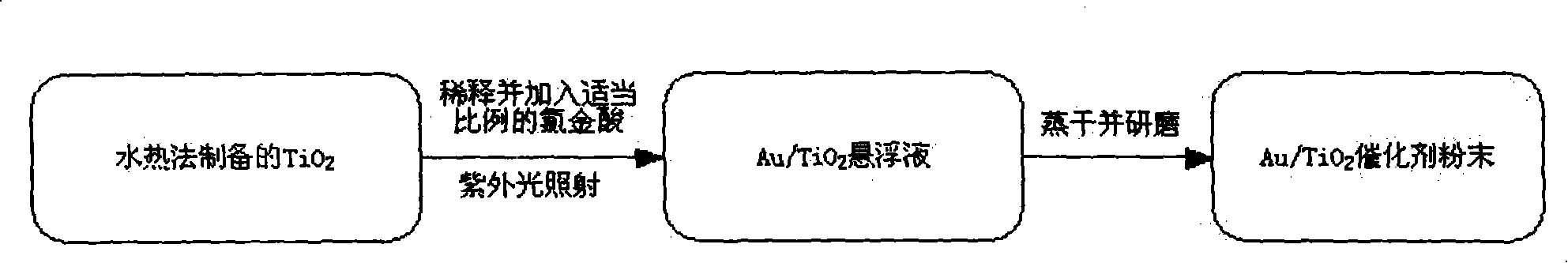
[0036] Preparation of Au / TiO by Photodeposition 2 The specific steps of the electro-oxidation catalyst can be found in figure 1 ,
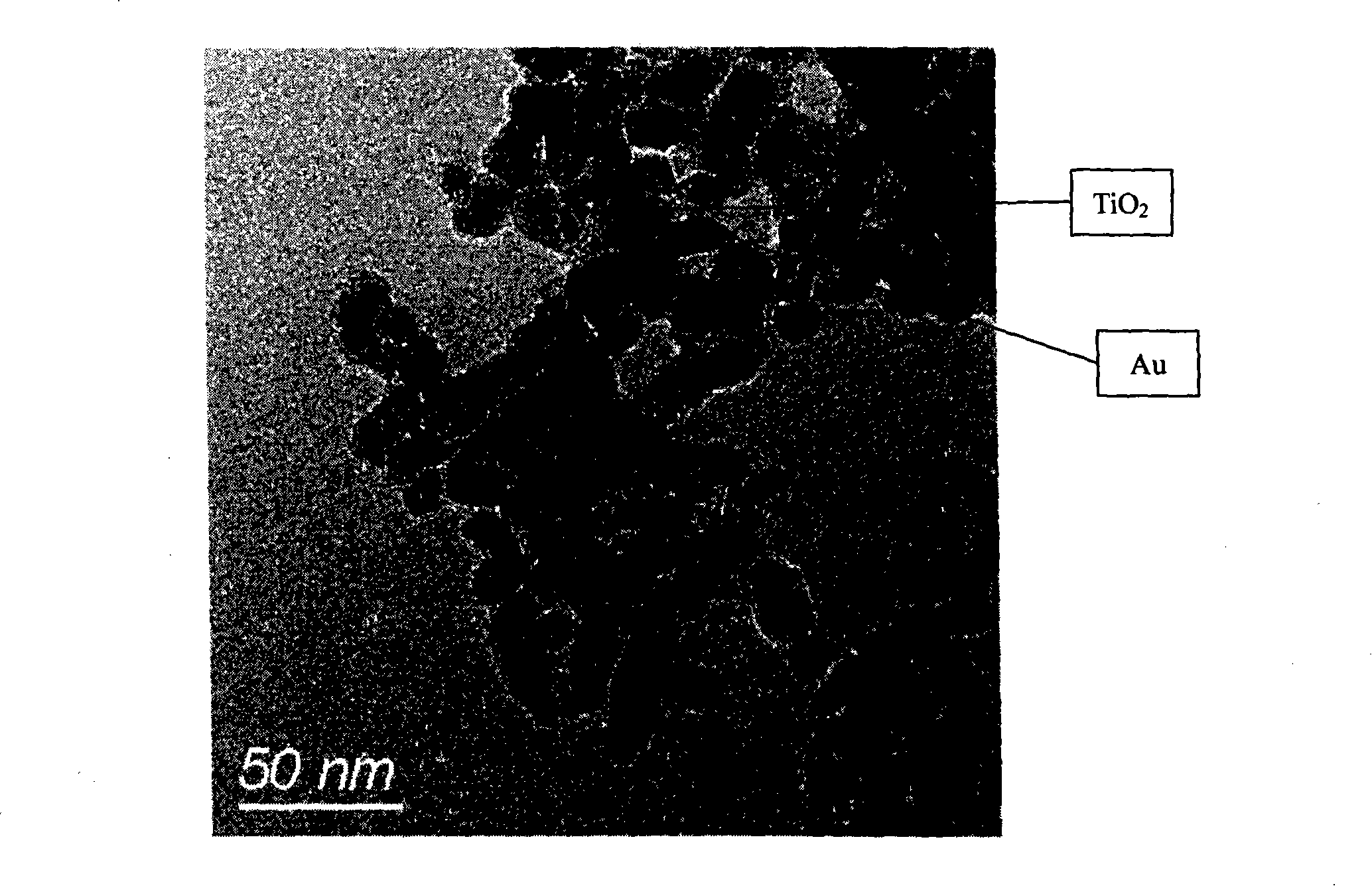
[0037] Take a certain amount of titanium dioxide prepared by the hydrothermal method, add an aqueous solution of chloroauric acid with a concentration of 2 mg / mL, and irradiate the suspension in step 1 under ultraviolet light for 2-5 minutes. The wavelength of ultraviolet light is 320-400 nanom...
Embodiment 2
[0040] The preparation of the electro-oxidation catalyst is as in Example 1, adding an aqueous solution of chloroauric acid with a concentration of 2 mg / mL, the wavelength of ultraviolet rays used is 320-400 nanometers, and the power is 0.85 mW / cm 2 , irradiated for 2 minutes to prepare 5wt% Au / TiO 2 Electrooxidation catalyst (in which the Au loading is 0.174mg / cm 2 ), the cathode uses a commercial Pt / C catalyst (where the Pt loading is 1mg / cm 2). The sides coated with the anode and cathode catalysts were facing the Nafion membrane, and hot-pressed at 1.5MPa and 140°C for 3min to form a membrane-electrode triple-in-one (MEA). In the test, the anode sodium borohydride solution uses NaBH 4 The content is 10wt%, which contains NaOH: 5wt%, NH 3 ·H 2 O: 5wt%; cathode hydrogen peroxide uses H 2 o 2 The content is 10wt%, which contains H 3 PO 4 : 5wt%. Assemble the three-in-one membrane electrode prepared above. The current collector plate is made of high-purity graphite. T...
Embodiment 3
[0042] The preparation of the electro-oxidation catalyst is the same as in Example 1, adding an aqueous solution of chloroauric acid with a concentration of 2 mg / mL, the wavelength of ultraviolet rays used is 320-400 nanometers, and the power is 0.85 mW / cm 2 , irradiated for 4 minutes to prepare 2wt% Au / TiO 2 Electrooxidation catalyst (in which the Au loading is 0.021mg / cm 2 ), the cathode uses a commercial Pt / C catalyst (where the Pt loading is 1mg / cm 2 ).
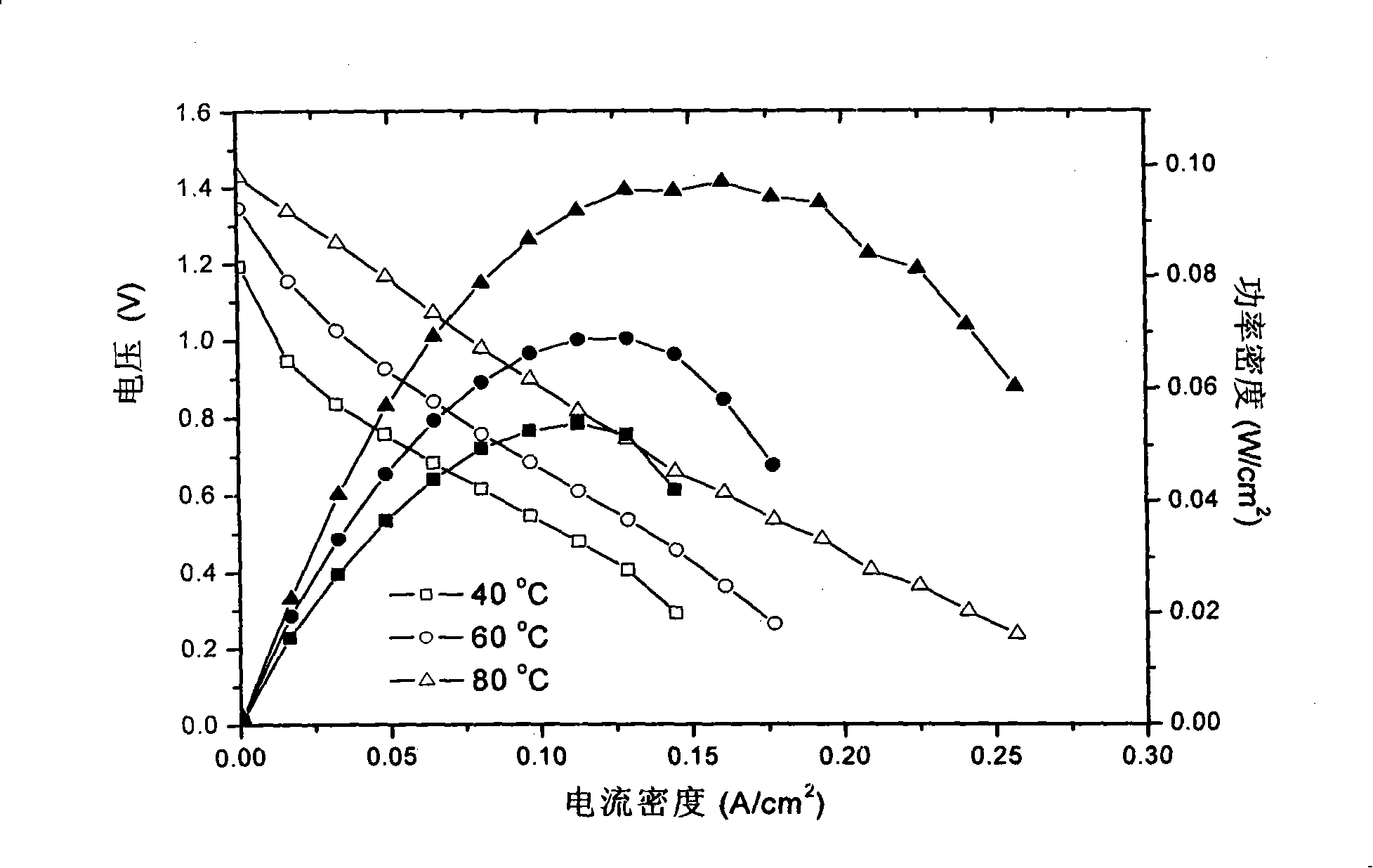
[0043] Obtain the discharge performance curve of battery under the solution concentration identical with embodiment 2 and test condition, its result sees Figure 4 . The battery is 100mA / cm at 80°C 2 Under the working voltage of 0.9V, at 200mA / cm 2 Get the maximum power density of 120mW / cm 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com