Small molecule peptides inhibitor of human heparinase
A technology of human heparanase and heptapeptide, which is applied in the field of biomedicine, can solve the problems of inapplicability in clinical practice and heterogeneous structure, and achieve the effect of potential medical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
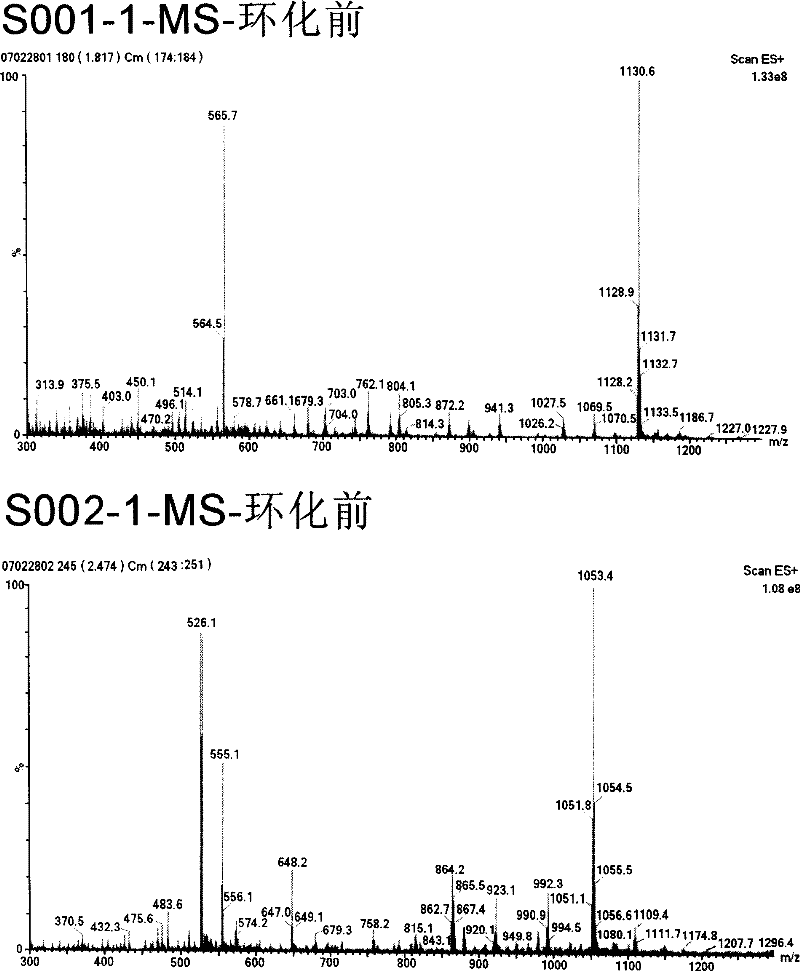
[0051] Example 1: Cloning and expression of human heparanase 8kD small subunit
[0052] 1. Construct the expression vector:
[0053] Apply the PCR method (primer P1: GGAATTC CATATG CAGGACGTCGTGGACCT (introduction of Ned I restriction site) (SEQ ID No.21) and P2: CCG CTCGAG TTCCTTCTTGGGATCG (introduction of Xho I restriction site) (SEQ ID No. 22).
[0054] reaction system:
[0055] 2×GC Buffer I 25μl
[0056] TaKaRa LA Taq 0.5 μl
[0057] dNTP Mixture 8μl
[0058] Template 1μl
[0059] Upstream primer 1.5μl
[0060] Downstream primer 1.5μl
[0061] Sterilized water to make up volume to 50 μl
[0062] Reaction conditions: 94°C pre-mutation for 5 minutes, followed by 30 cycles of denaturation at 94°C for 1 minute, annealing at 57°C for 1 minute, extension at 72°C for 1 minute, and final extension at 72°C for 10 minutes). DE3) The engineered strains were constructed and preserved by our laboratory, among which the pGEX-2PK vector is a product of GE Healthcare, and the...
Embodiment 2
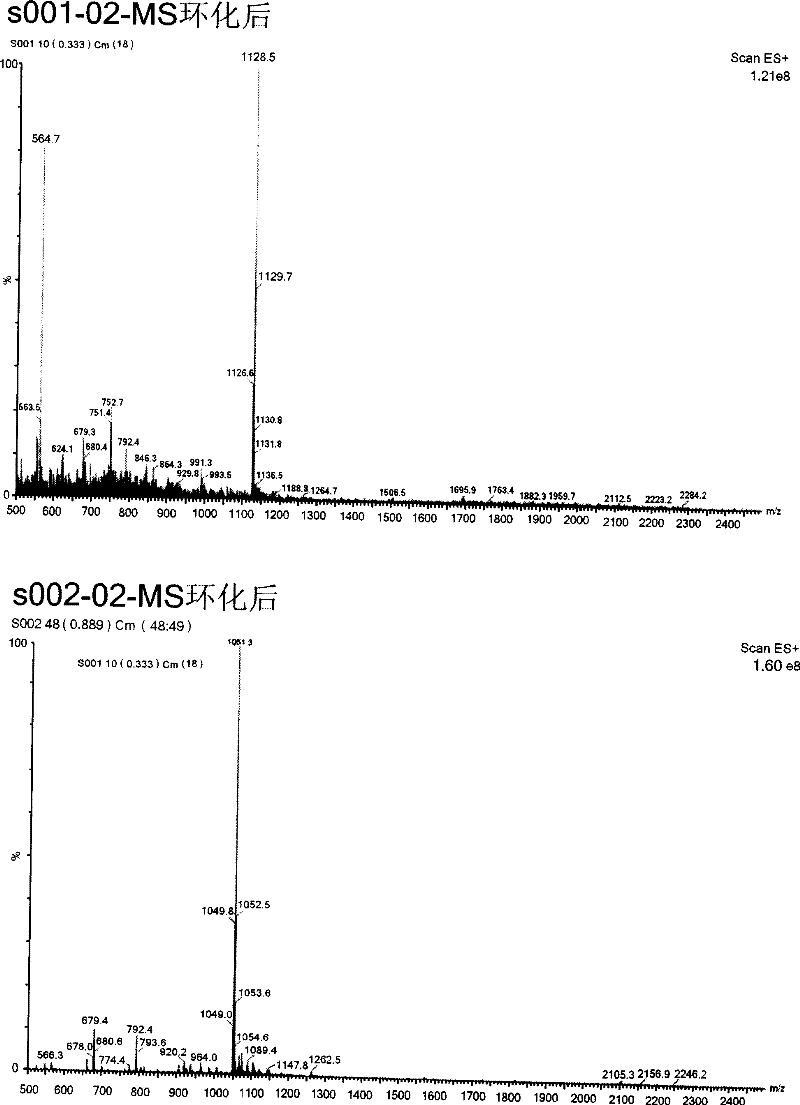
[0079] Example 2: Screening of Human HPA Small Subunit Inhibitors
[0080] 1. On the basis of obtaining the human HPA small subunits whose purity and concentration meet the requirements, use the human HPA small subunits as the target molecular screening phage display random cyclic heptapeptide library (New England Biolabs company kit product), after five rounds screening, the recovery rate increased by about 10 3 times. From the plate of the last round of screening, 150 phage plaques were randomly picked and placed in the ER2738 culture in the logarithmic growth phase (New England Biolabs kit product), cultured with vigorous shaking at 37°C for 4.5h, and then centrifuged at 4°C. Clear, save the original phage species, and then prepare 150 corresponding monoclonal samples, select positive clones by ELISA detection, extract phage single-stranded DNA, and send it for sequencing. According to the DNA sequencing results, the amino acid sequences of the corresponding binding peptide...
Embodiment 3
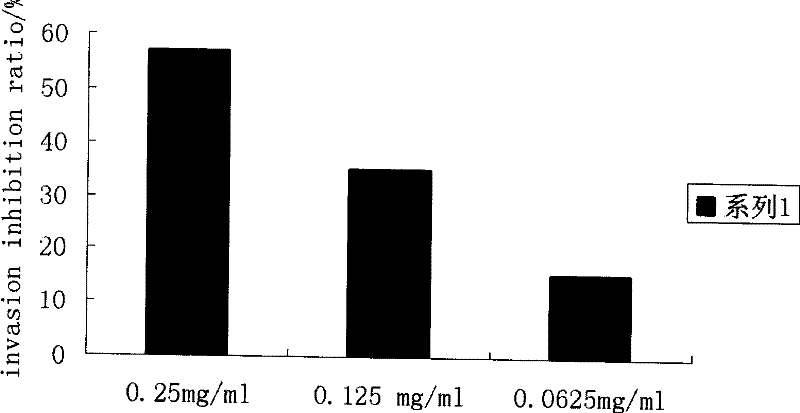
[0094] Example 3: Detection of C7-1 and C7-5 activities using HepG-2 cells in vitro reconstituted basement membrane invasion assay
[0095] 1. Method
[0096] 1. Digest HepG-2 cells in the logarithmic growth phase (gifted by Mr. Gong Xin, Institute of Bioengineering, Academy of Military Medical Sciences) with trypsin (product of Amresco), add serum-containing medium, centrifuge, discard the culture medium, and wash with PBS for 1 resuspend with 0.1% BSA-serum-free DMEM medium (product of HyClone Company), and adjust the final concentration of HepG-2 cells to 1×10 6 / mL. Add 10% FBS-DMEM medium to the 24-well plate (lower chamber), 600 μL / well; use sterile tweezers to transfer the cell culture pool covered with Matrigel gel into the 24-well plate (lower chamber).
[0097] 2. Take 160 μL of HepG-2 cell suspension and add it to the cell culture pool; add different concentrations of C7-1 and 0.25 mg / mL C7-5 respectively, and add an equal volume of 0.1% BSA-serum-free DMEM medium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com