Broccoli protein-derived ace and dpp-iv inhibitory peptides, preparation method and application thereof
A technology derived from DPP-IV and protein, which is applied in the field of broccoli protein-derived ACE and DPP-IV inhibitory peptides and their preparation, can solve problems such as the impact on kidney function, and achieve the effects of strong activity, simple structure, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
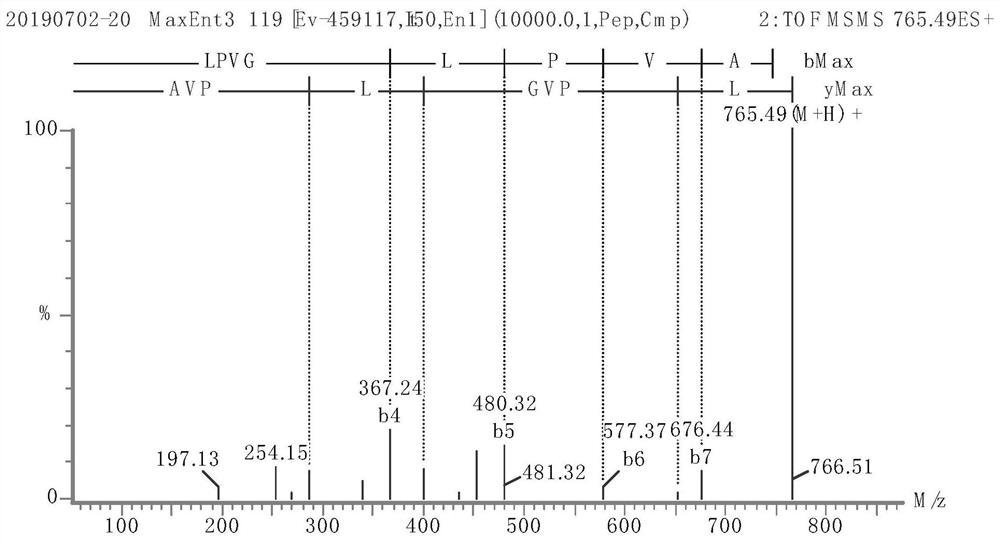
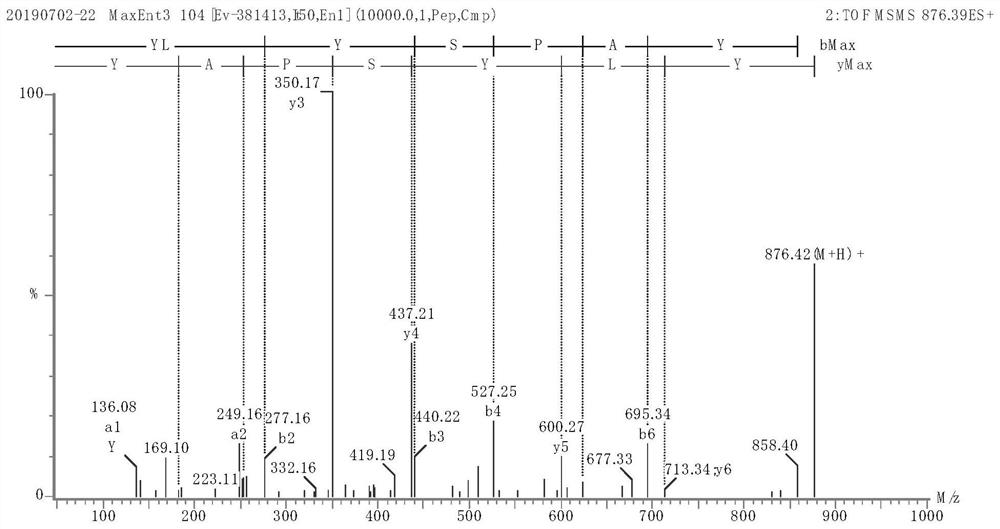
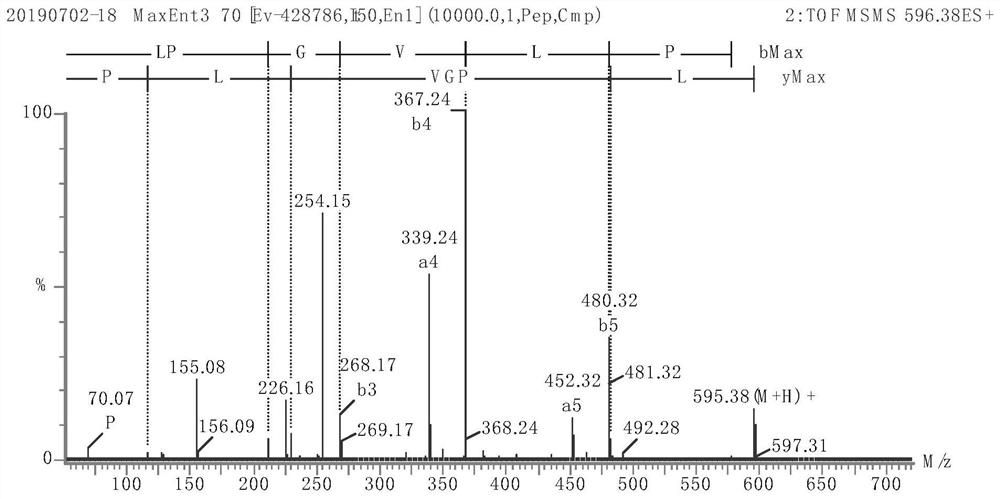
Image
Examples
Embodiment 1
[0044] (1) Squeeze fresh broccoli stems and leaves as raw materials, heat the obtained juice to 90°C, keep it warm for 10 minutes, centrifuge the sediment, and dry it to obtain the crude broccoli protein extract;
[0045] (2) Enzymatic preparation of polypeptide extracts from broccoli stems and leaves
[0046] Use broccoli protein crude extract as raw material, add distilled water according to the mass ratio of solid to liquid 1:20, and add Aclase alkaline protease with 2.0% broccoli protein crude extract mass under the condition of temperature 60°C and pH 8.0, and enzymatically hydrolyze for 3 hours Finally, inactivate the enzyme, cool down, centrifuge, rotate evaporate, concentrate and then freeze-dry to obtain the broccoli polypeptide extract;
[0047] (3) Ultrafiltration and gel filtration of broccoli peptide extract
[0048] The broccoli polypeptide extract was ultrafiltered with Valflow 50, and then separated with a G-15 gel column (Sephadex G-15). The elution condition...
Embodiment 2
[0055] Experimental steps include:
[0056] 1. Detection method of angiotensin-converting enzyme (ACE) inhibitory activity
[0057] Add 80uL 5mmol / L HHL (hippuryl-histidyl-leucine) (dissolved in HEPES buffer, pH8.3) and 30uL sample solutions of different concentrations (dissolved in double-distilled water) in a centrifuge tube, mix After placing in a water bath at 37°C for 5min, add 40uL 0.025U / mL ACE (dissolved in HEPES buffer, pH 8.3), incubate at 37°C for 1h, then add 150uL 1M hydrochloric acid to terminate the reaction. The blank group added hydrochloric acid while adding ACE, the control group used 30ul double distilled water instead of the sample solution, and captopril (10ng / mL) was used as a positive control. After the reaction was completed, the content of hippuric acid (HA) in the sample was detected by RP-HPLC, and the content of hippuric acid in the detection sample was calculated by comparing with the peak area of the standard hippuric acid. Chromatographic co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume ratio | aaaaa | aaaaa |
| volume ratio | aaaaa | aaaaa |
| volume ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com