Preparation of nano crystal fibre felt of water-insoluble medicament
A nanocrystal, water-insoluble technology, applied in the direction of drug delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of increasing the difficulty of drug approval work, a large number of carrier materials, and low bioavailability and other problems, to achieve the effect of improving the oral bioavailability of drugs, simple preparation methods, and a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of ibuprofen-polyvinylpyrrolidone drug-loaded nanofiber mats:
[0042] Dissolve 10 g of ibuprofen in 100 mL of ethanol, then add 20 g of polyvinylpyrrolidone under stirring conditions, continue stirring for 2 hours after the addition, and then use an ultrasonic processor (500W) for ultrasonic degassing for 15 minutes.
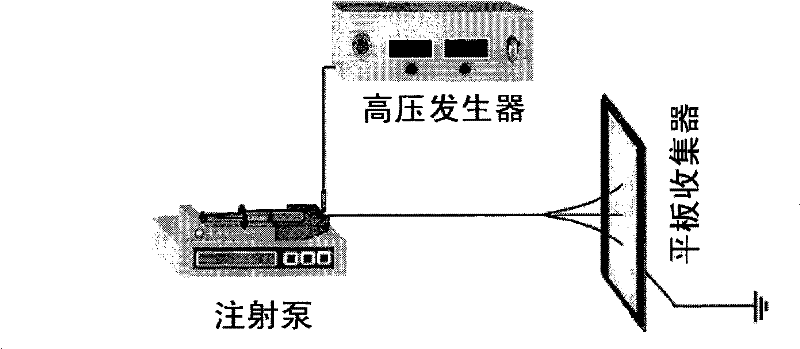
[0043] Pour the prepared solution into the solution reservoir (5mL syringe), use the flattened No. 6 injection needle as the capillary for jetting fine flow, connect the positive electrode of the high-voltage power supply, and connect the negative electrode with an aluminum foil receiving plate. Pump control, electrospinning was carried out according to the following conditions: the flow rate was 1.0mL h -1 , the distance between the receiving plate and the spinneret is 15cm, the voltage is 12kV, the ambient temperature is 20°C, and the ambient humidity is 65%.
Embodiment 2
[0045] Preparation of ibuprofen nanosuspension:
[0046] Put the aluminum foil receiving plate receiving the drug-loaded fiber into an electric vacuum drying oven at 35°C and a vacuum of 320Pa to dry for 24 hours, then remove the fiber mat and dissolve it in water.
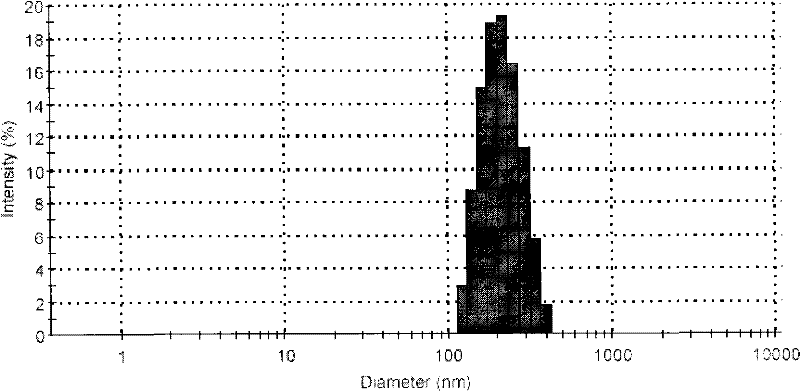
[0047] Properly dilute the nano-suspension, measurement conditions: T=25°C, λ=633nm, He-Ne excitation light source, incident angle 90°. The particle size and polydispersity index of nanoparticles were measured on a laser particle size analyzer. The result is as figure 2 shown.
Embodiment 3
[0049] Stability investigation of nano-suspension:
[0050] The nano-suspension of Example 2 was kept airtight at room temperature, and the particle size of the nano-suspension was measured within a set time interval. It can be seen from Table 1 that the particle size of the nano-suspension increased from 207.2nm to 227.5nm in the first month, and the polydispersity index (PDI) increased from 0.180 to 0.242, and there was no significant change. It shows that polyvinylpyrrolidone provides greater steric hindrance, and at the same time, the electrostatic repulsion between nanoparticles prevents the agglomeration of nanoparticles, and together maintains the stability of nano-suspension.
[0051] Table 1 Nanosuspension Stability Experimental Results
[0052]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com