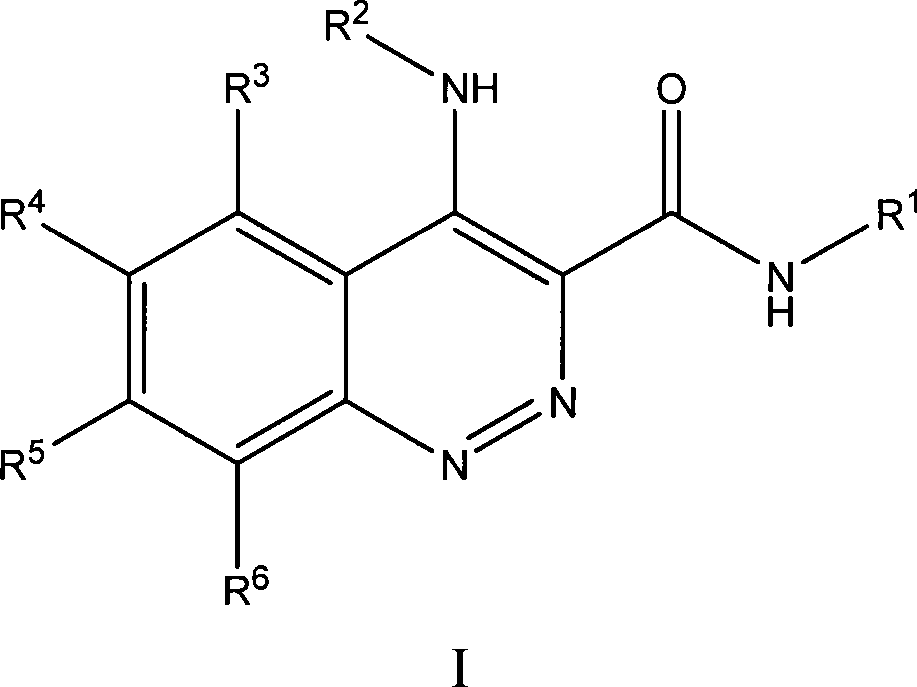
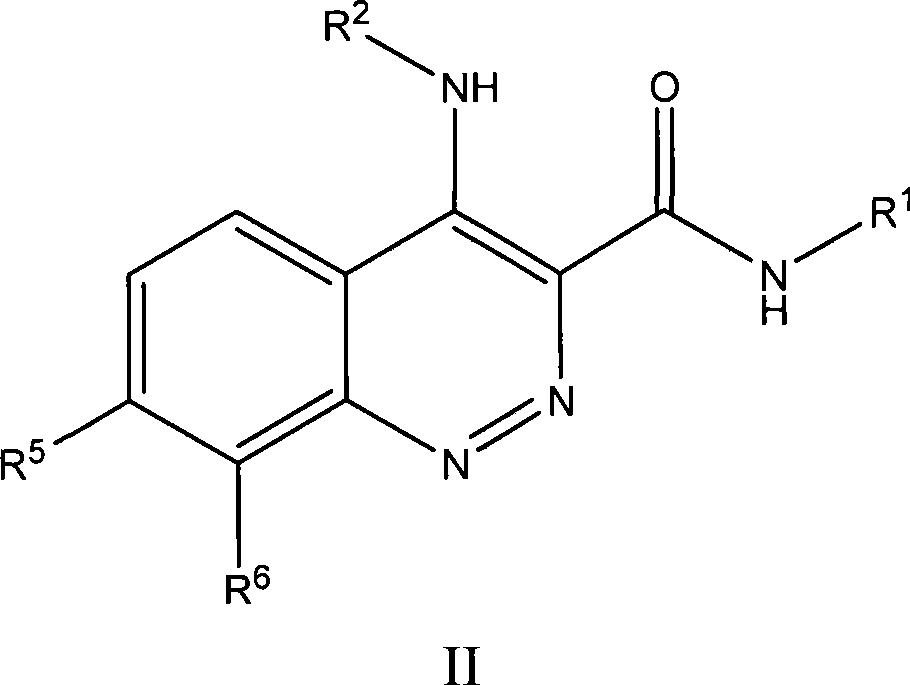
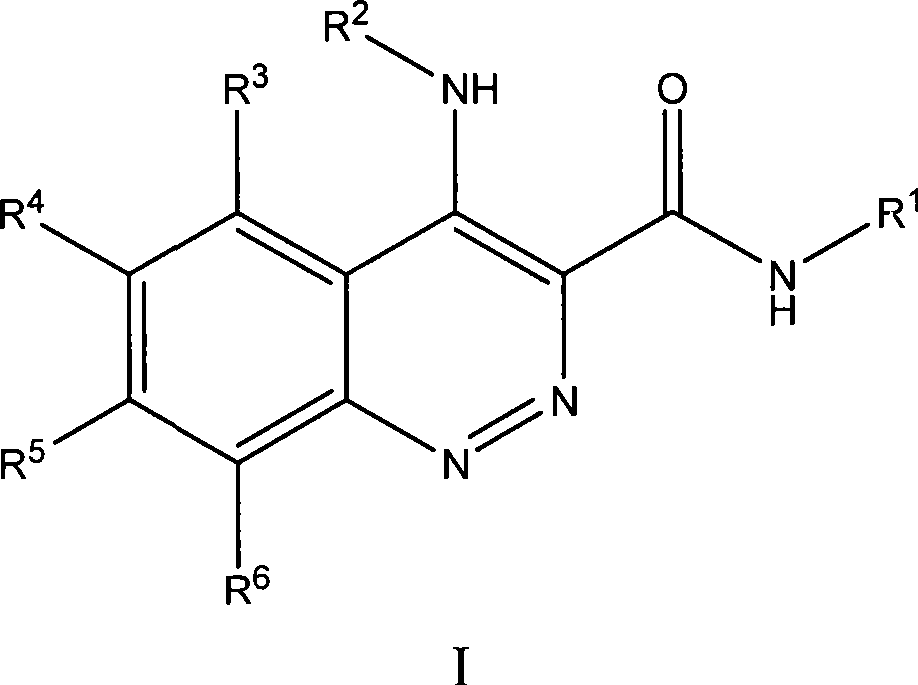
Substituted cinnoline derivatives as GABAA-receptor modulators and method for their synthesis
A precursor, compound technology, applied in the field of treatment and/or prevention of anxiety disorders, cognitive disorders and/or mood disorders, which can solve problems such as drug incompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0573] Example 1: 4-amino-7-fluoro-8-phenyl-N-propyl-cinnoline-3-carboxamide
[0574] Using Method F, 4-Amino-7-fluoro-8-iodo-N-propyl-cinnoline-3-carboxamide (291 mg, 0.78 mmol) and phenylboronic acid (379 mg, 3.11 mmol) were reacted (refluxed for 4 hours) ) to afford the title compound (65 mg, 26% yield) as a white solid. 1 H NMR (300MHz, CDCl 3 )δ 8.52 (bs, 1H), 7.89 (dd, J = 9.2, 4.6Hz, 1H), 7.42-7.60 (m, 6H), 3.45 (apparent q, J = 6.6Hz, 2H), 1.65 (apparent Sextet, J = 7.2 Hz, 2H), 1.00 (t, J = 7.4 Hz, 3H). MS APCI, m / z=325 (M+H). HPLC 1.92min.
Embodiment 2
[0575] Example 2: 4-amino-7-chloro-8-phenyl-N-propyl-cinnoline-3-carboxamide
[0576] Using Method F, 4-Amino-7-chloro-8-iodo-N-propyl-cinnoline-3-carboxamide (184 mg, 0.47 mmol) and phenylboronic acid (229 mg, 1.89 mmol) were reacted (40 hours at reflux ) to afford the title compound (90 mg, 56% yield) as a white solid. 1 H NMR (300MHz, CDCl 3 )δ 8.49 (bs, 1H), 7.82 (d, J = 9.0Hz, 1H), 7.75 (d, J = 9.0Hz, 1H), 7.42-7.55 (m, 5H), 3.43 (obvious q, J = 6.6 Hz, 2H), 1.63 (clear sextet, J = 7.2 Hz, 2H), 0.98 (t, J = 7.4 Hz, 3H). MS APCI, m / z=341 (M+H). HPLC 2.04min.
Embodiment 3
[0577] Example 3: 4-amino-7-methoxy-8-phenyl-N-propyl-cinnoline-3-carboxamide
[0578]Using Method F, 4-Amino-7-methoxy-8-iodo-N-propyl-cinnoline-3-carboxamide (311 mg, 0.81 mmol) and phenylboronic acid (394 mg, 3.24 mmol) were reacted (reflux overnight) to afford the title compound (140 mg, 52% yield) as a white solid. 1 H NMR (300MHz, CDCl 3 )δ 8.51(bm, 1H), 7.90(d, J=9.2Hz, 1H), 7.52(d, J=9.2Hz, 1H), 7.36-7.50(m, 5H), 3.92(s, 3H), 3.43 (clear q, J=6.4Hz, 2H), 1.63 (clear sextet, J=7.2Hz, 2H), 0.98 (t, J=7.4Hz, 3H). MS APCI, m / z=337 (M+H). HPLC 1.76min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com