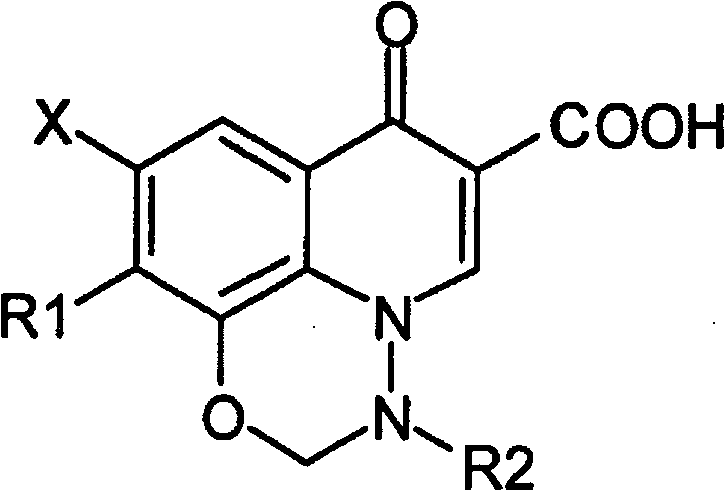
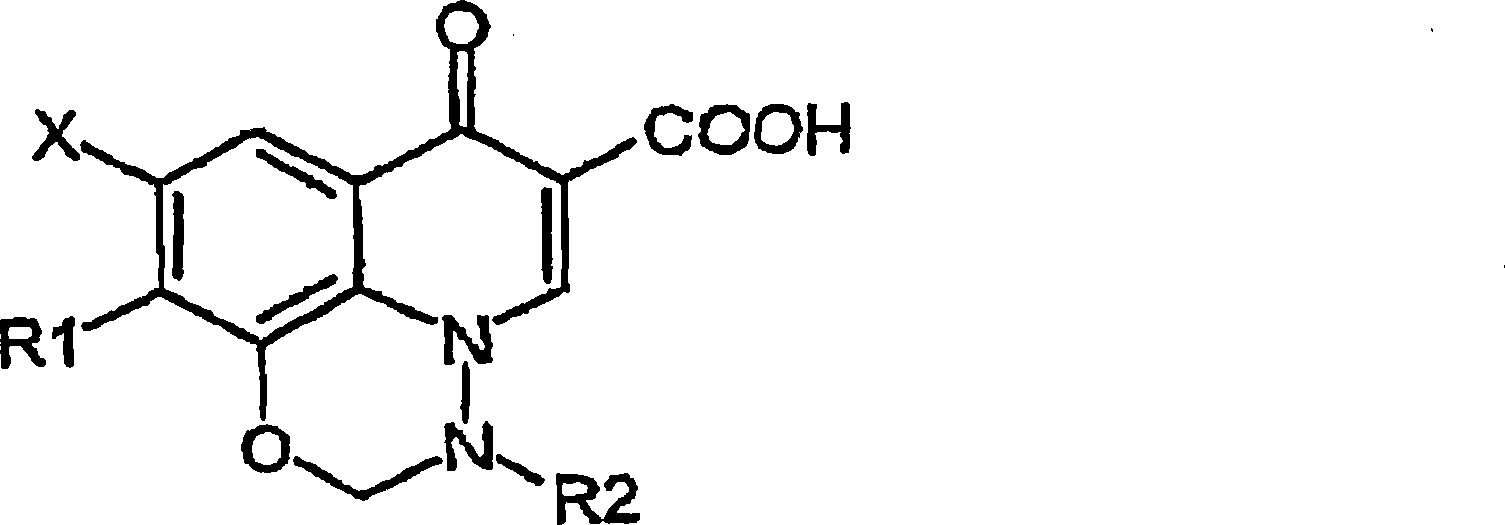
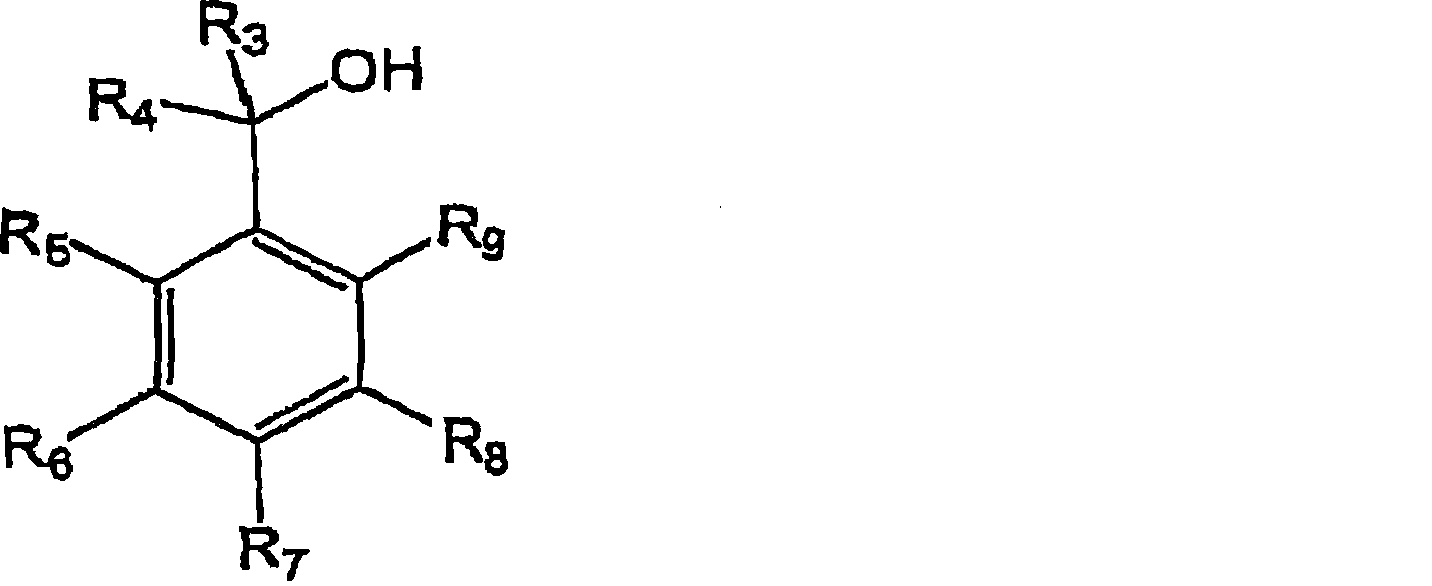Anti-infective solution comprising a compound of pyrido(3,2,1-ij)-benzoxadiazine type
A compound and anti-infection technology, applied in the direction of anti-infective drugs, medical preparations containing active ingredients, medical preparations with non-active ingredients, etc., can solve problems such as exerting sufficient effects, inapplicable and incapable of all molecular types, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] The solutions described in Table 1 below were prepared:
[0077]
1st copy comparative example Marbofloxacin (unit g) 15.00 10.00 Gluconolactone (unit g) 8.11 8.00 demineralized water qsp 100ml 100ml
[0078] Pigs with a weight of 70-80kg received intramuscular injection, and the injection solution was a single comparative solution in the right column or the first solution in the left column, and the dose was 8ml / kg. One week after dosing, local tolerance (damage) was checked.
[0079] Injection of the first solution presents a lesion volume of approximately 9 cm 3 , while the injection solution of the comparative example exhibits up to 32cm 3 damage volume.
[0080] The results indicated that the concentrated solution of marbofloxacin exhibited better local tolerance compared to the control solution.
Embodiment 2
[0082] The following solutions were prepared:
[0083]
1st copy 2nd copy 3rd copy 4th copy Marbofloxacin 20.00g 20.00g 20.00g 20.00g Gluconolactone 19.00g 19.00g 17.00g 17.00g Benzyl alcohol - 2.00g - 2.00g demineralized water qsp100ml qsp100ml qsp100ml qsp100ml stability at low temperature 24 hours >7 days* 24 hours >7 days*
[0084] * After 7 days, no precipitation was found.
[0085] "Stability at low temperature" refers to the time for each solution to stand at +4 to +8°C until a precipitate forms.
[0086] The above data clearly show the improved stability of the solutions of the invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com