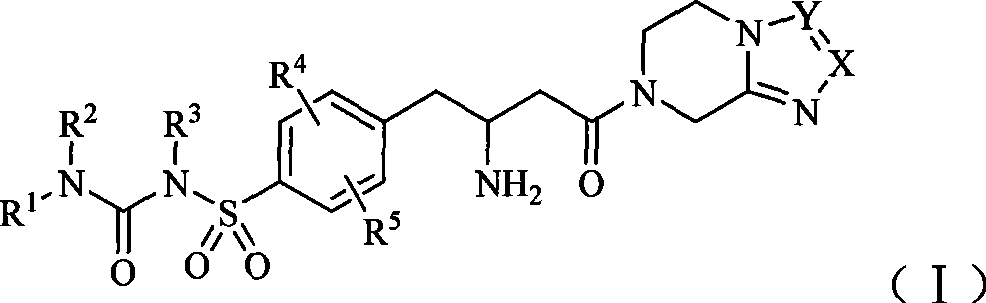
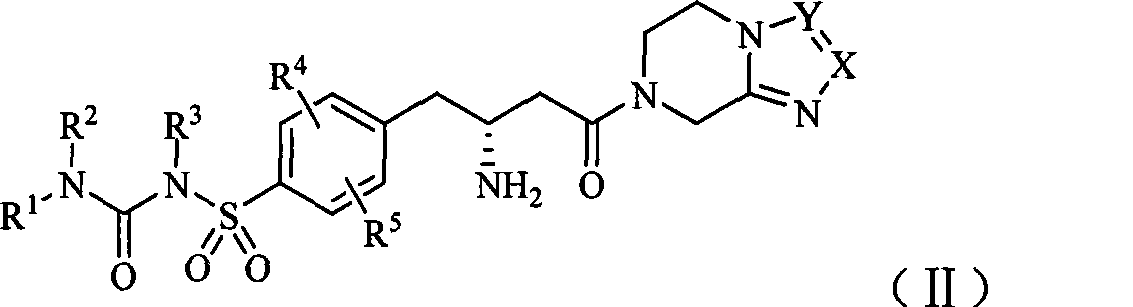
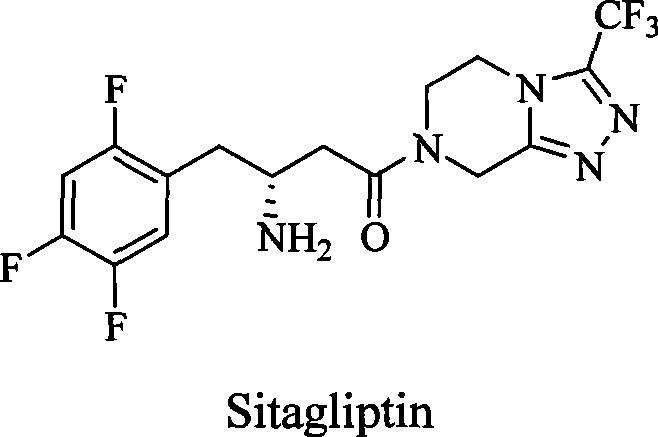
Dipeptidase-IV inhibitor sulfonyl urea derivates
A technology of compounds and compositions, applied in the field of dipeptidase-IV inhibitor sulfonylurea derivatives, capable of solving problems such as poor drug activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] Example 1 (2R, 5S)-2,5-dihydro-3,6-dimethoxy-2-[(2,5-difluorophenyl)methyl]-5-isopropylpyrazine preparation
[0153] Dissolve 9.2g (50mmol) (2S)-(+)-2,5-dihydro-3,6-dimethoxy-2-isopropylpyrazine in 300ml tetrahydrofuran, then at -78°C for 30min Add 24ml (60mmol, 2.5N) of n-butyllithium / n-hexane solution dropwise, keep stirring for 30min, then dropwise add 8.9g (55mmol) of 2,5-difluorobenzyl chloride in 50ml of tetrahydrofuran cold solution, -78 Stir the reaction at ℃ for 5 hours, add 100ml of water at -78℃ to quench the reaction, concentrate the reaction solution, add ethyl acetate and 1N hydrochloric acid to the residue, separate the water layer, extract three times with ethyl acetate, combine the organic layers, wash with salt, and anhydrous Dry over magnesium sulfate, concentrate under reduced pressure, and purify on a silica gel column (the eluent is a mixture of ethyl acetate and cyclohexane) to obtain 10.8 g of the target compound as a solid, yield: 69.4%.
Embodiment 2
[0154] Example 2 Preparation of (R)-2-(tert-butoxycarbonyl)amino-3-(2,5-difluorophenyl)propionic acid methyl ester
[0155] 15.5g (50mmol) (2R,5S)-2,5-dihydro-3,6-dimethoxy-2-[(2,5-difluorophenyl)methyl]-5-isopropyl Dissolve pyrazine in 100ml of acetonitrile, then add 100ml (1N) of hydrochloric acid dropwise, stir the reaction solution at room temperature for 24h, add methanol, concentrate to dryness, repeat this operation three times, and then repeat once with toluene to obtain a solid, which is used in 400ml After dichloromethane dissolves, slowly add 50g of triethylamine, then slowly add 24g (110mmol) (Boc) 2 O, the reaction solution was stirred at room temperature for 8 hours, the solid generated by the reaction was filtered off, the filtrate was diluted with dichloromethane, washed with 1N HCl solution and saturated brine respectively, dried over anhydrous magnesium sulfate, purified by a silica gel column, and the eluent was acetonitrile and petroleum The ether mixtur...
Embodiment 3
[0156] Example 3 Preparation of (R)-2-(tert-butoxycarbonyl)amino-3-(2,5-difluorophenyl)propionic acid
[0157] Add 200ml tetrahydrofuran to 9.5g (30mmol) of (R)-2-(tert-butoxycarbonyl)amino-3-(2,5-difluorophenyl)propionic acid methyl ester, cool in an ice bath, and then dropwise add 2.15g (90mmol) of lithium hydroxide in 200ml aqueous solution, the reaction solution was stirred at room temperature for 15h, concentrated under reduced pressure, the residue was dissolved in ethyl acetate, washed with saturated sodium bicarbonate and brine, dried over anhydrous magnesium sulfate, and evaporated to obtain 6.9g Solid, yield: 76.2%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap