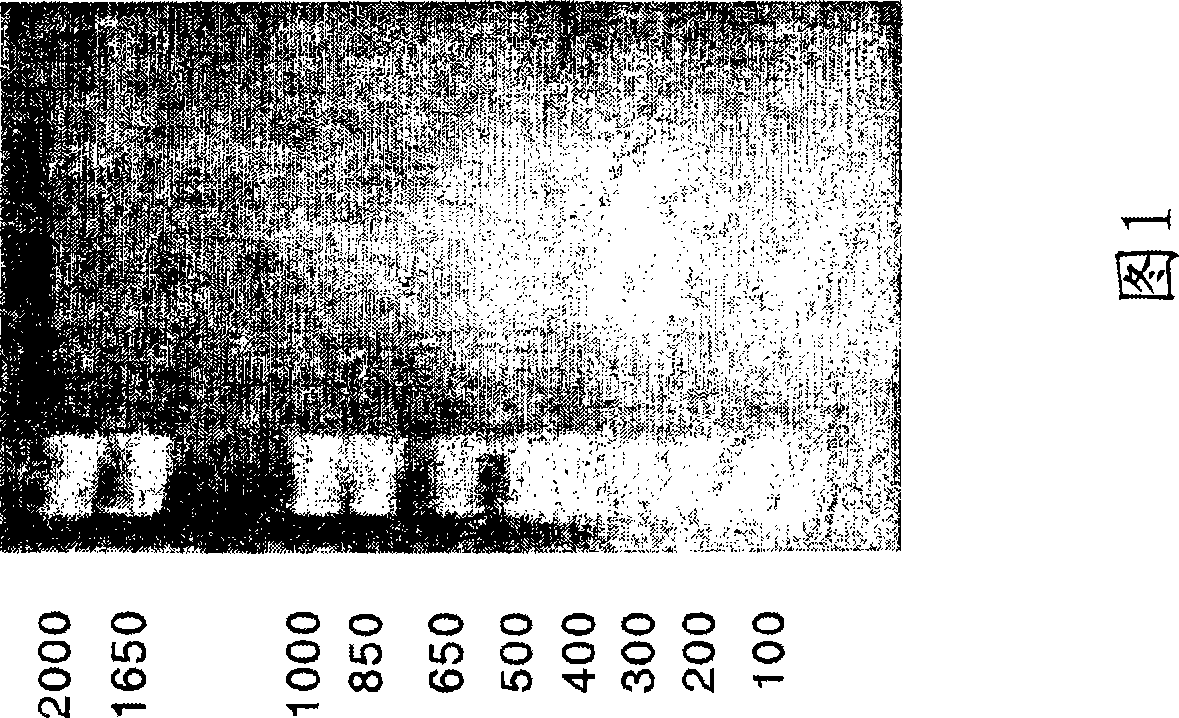Cathepsin propeptide and uses thereof
A technology of cathepsin and protease, applied in the field of cathepsin propeptide and its application, can solve the problems of low specificity and unreliability
Inactive Publication Date: 2009-05-13
FUSION ANTIBODIES
View PDF10 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The clinical use of this compound is not reliable due to low specificity, inhibition of proteases in normal tissues and possible reactivity to bystander proteins (Turk et al., 2004)
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
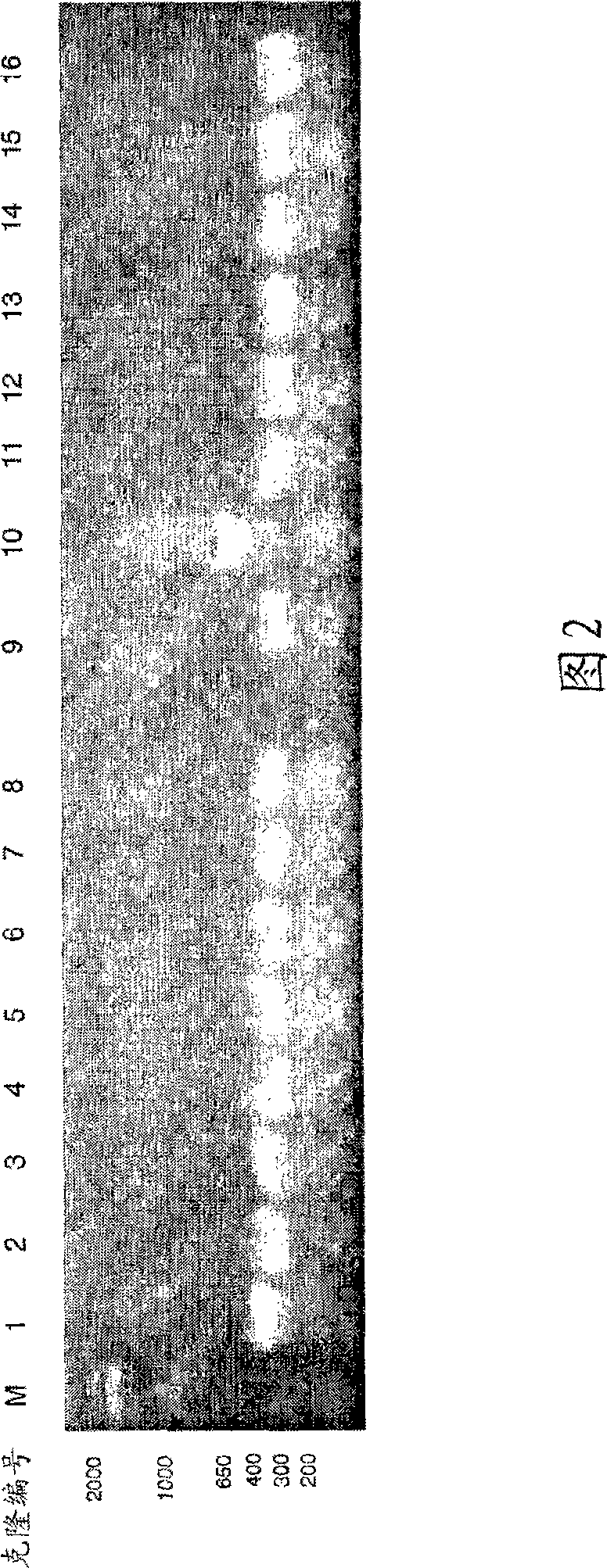
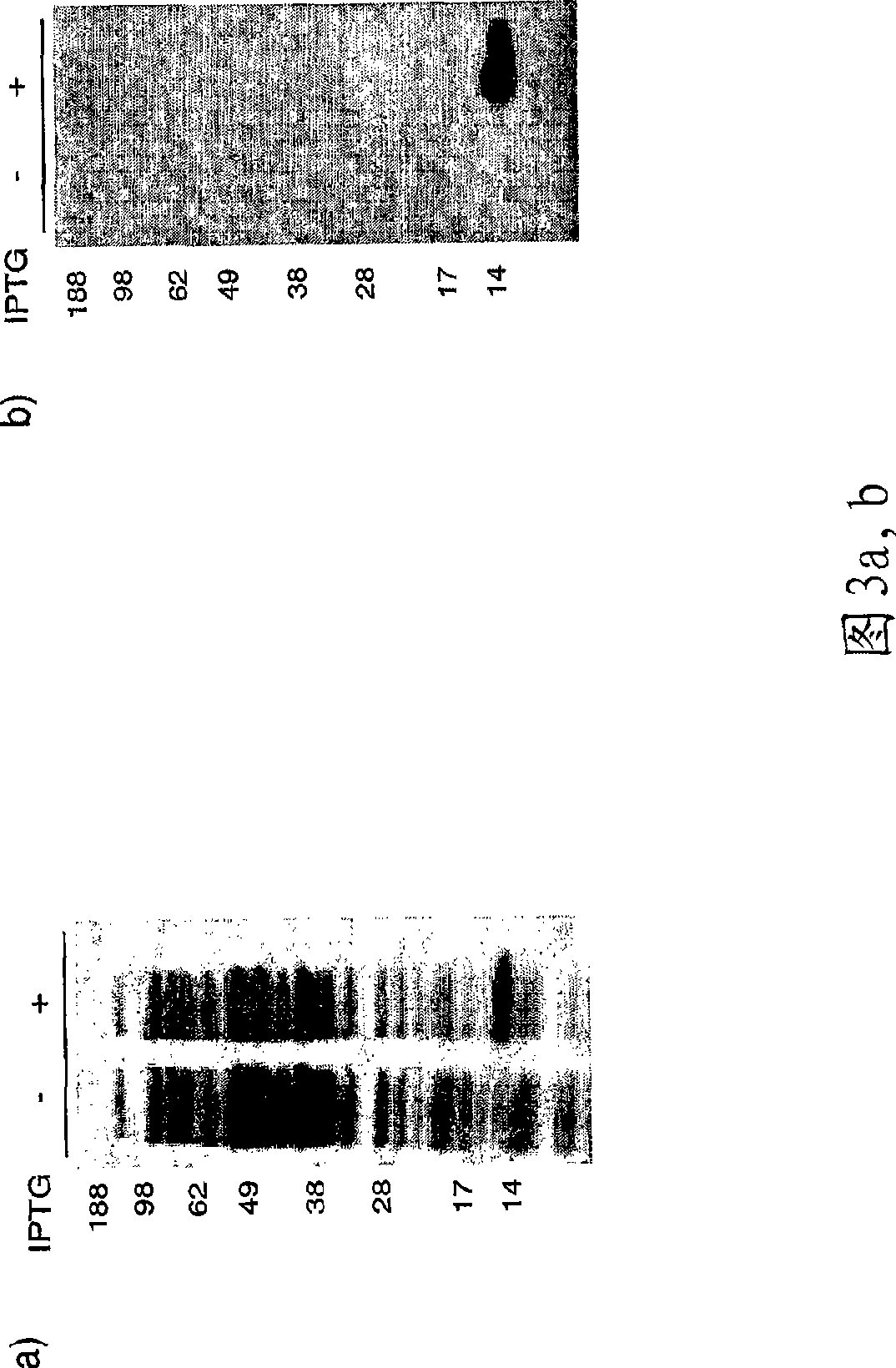
[0132] Materials and methods
[0133] Cloning and expression of CatSPP
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Login to View More
Abstract
A method of inhibiting activity of a cathepsin L-like protease in cells or tissue and the use of the method in the treatment of disease such as cancer and inflammatory diseases is described. The method comprises administration of a cathepsin propeptide or a nucleic acid encoding a cathepsin propeptide. In particular embodiments, the propeptide is a Cathepsin S propeptide. Further, the use of propeptides having an Fc portion is described.
Description
Technical field [0001] This application is about a peptide and its application in therapeutic methods. Specifically, it relates to a cathepsin propeptide, its production method and the application of the propeptide. Background technique [0002] Proteases are a large group of proteins that comprise approximately 2% of all gene products (Rawlings and Barrett, 1999). Proteases catalyze the hydrolysis of peptide bonds and are extremely important for the normal function of all cells and organisms. Proteolytic processing events are important in a wide range of cellular processes including bone formation, wound healing, angiogenesis, and apoptosis. [0003] Lysosomal cysteine proteases were originally thought to be enzymes responsible for non-selective degradation of proteins in lysosomes. Usually related to lysosomal location, these proteases were initially thought to be involved only in non-selective degradation of endosomal compartment proteins. However, they are now known to be r...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K38/48A61P9/10A61P25/28A61P35/00
CPCA61K38/4873A61P11/06A61P25/28A61P29/00A61P35/00A61P37/02A61P43/00A61P9/10
Inventor 克里斯多佛·斯科特罗伯塔·波尔登吉姆·约翰斯顿马克·麦克理菲利普·斯诺迪理查德·别克
Owner FUSION ANTIBODIES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com