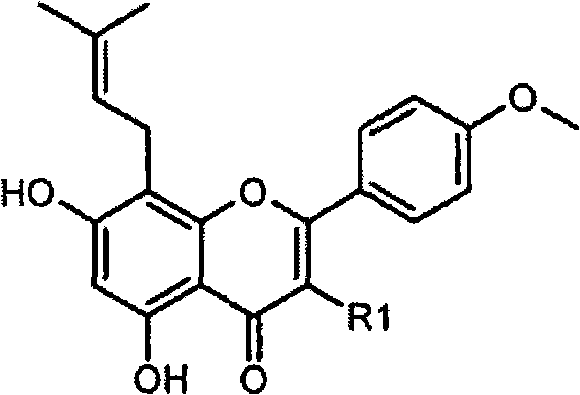
Method for preparing icariside II, cosmetic composition containing the same and the use thereof for skin whitening
A cosmetic composition, icariin technology, applied in the direction of medical preparations containing active ingredients, cosmetics, cosmetic preparations, etc., can solve the problems of insufficient whitening effect, use restrictions, etc., to achieve excellent skin whitening Effect, effect of suppressing melanin production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of Icariside II by Extraction
[0040] 2 kg of dried leaves of Epimedium korean were added to 6 liters of hexane, and extracted three times at room temperature with stirring. 1 kg of defatted plant leaves was added to 4 liters of methanol, extracted three times under reflux, and then settled at 15 °C for 1 day. Afterwards, the settled material is filtered through a filter cloth and centrifuged into a residue and a filtrate. The filtrate was concentrated under reduced pressure. The obtained extract was suspended in water and extracted 5 times with ether to remove the pigment, and the aqueous layer was extracted once with 500 ml of 1-butanol. The resulting entire 1-butanol layer was concentrated under reduced pressure to obtain a 1-butanol extract, which was then dissolved in a small amount of methanol. This solution was added to a large amount of ethyl acetate, and the formed precipitate was dried, thereby obtaining an extract containing icariin...
Embodiment 2
[0041] Example 2: Preparation of Icariside II Using Cellulase
[0042] 10 g of icariin was dissolved in 500 ml of 0.1 M acetate buffer solution (pH 4.5), and 0.5 g of cellulase (Sigma) was added thereto. The solution was stirred in a water bath at 37°C for 48 hours while it was checked periodically by thin layer chromatography. When icariin completely disappeared, the reaction solution was heated in hot water (80-100° C.) for 10 minutes to terminate the reaction, and then concentrated under reduced pressure to remove the solvent. The residue was added to 200 ml of ethanol, the solution was stirred three times, and filtered to remove a precipitate, and the filtrate was concentrated under reduced pressure to obtain a crude product. The crude product was separated by silica gel column chromatography (chloroform:methanol=8:1-4:1), thereby obtaining 7.5 g of icariin II.
Embodiment 3
[0043] Example 3: Preparation of Icariside II using β-glucosidase
[0044] 10 g of icariin was dissolved in 500 ml of 0.1 M acetate buffer solution (pH 5.5), and 0.5 g of β-glucosidase (Sigma) was added thereto. The solution was stirred in a water bath at 25°C for 48 hours while periodically checking it by thin layer chromatography. When icariin completely disappeared, the reaction solution was heated in hot water (80-100° C.) for 10 minutes to terminate the reaction, and then concentrated under reduced pressure to remove the solvent. The residue was added to 200 ml of ethanol, the solution was stirred three times, and filtered to remove the precipitate. The filtrate was concentrated under reduced pressure to obtain a crude product. The crude product was separated by silica gel column chromatography (chloroform:methanol=8:1-4:1), thereby obtaining 6.9 g of icariin II.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com