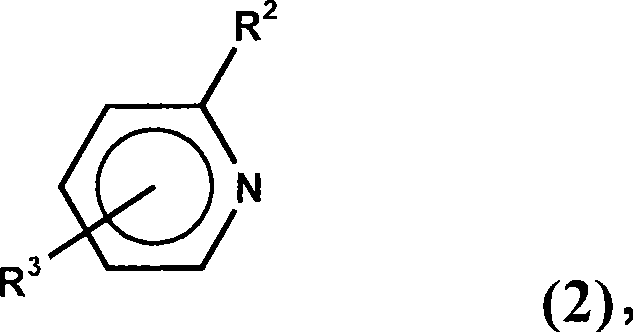
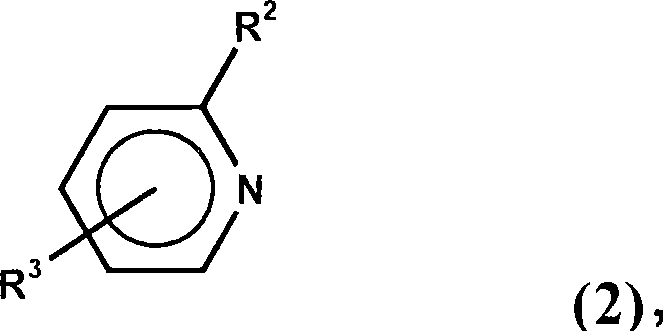
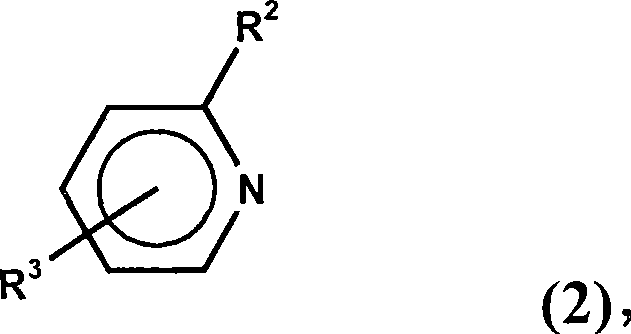
Dialkylborane amine complexes
A technology of alkylborane amines and complexes, which is applied in the field of new dialkylborane amine complexes, which can solve problems such as difficult large-scale processing, steric volume hindering coordination, and not being separated.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Preparation of 9-BBN-5-ethyl-2-picoline complex in THF:
[0067] 1.21 g (0.01 mol) of 5-ethyl-2-methylpyridine was added to 20 ml of a 0.5M 9-BBN (0.01 mol) solution in THF at 0-5°C within 15 minutes. of the reaction mixture 11 B NMR spectrum no longer shows the signal of 9-BBN at 27.8ppm, but a new doublet (80Hz) signal appears at d=-1.3, attributed to 9-BBN-5-ethyl-2-picoline Complexes. Part of the THF was removed under vacuum to leave a concentrate, about 60 wt% 9-BBN-5-ethyl-2-picoline complex. 11 B NMR spectrum shows the product as a broad singlet at d = -0.8 (98% purity).
Embodiment 2
[0068] Example 2: Preparation of 9-BBN-5-ethyl-2-picoline complex in hexane:
[0069] 49.7 g (0.41 mol) of 5-ethyl-2-methylpyridine was added to 820 ml of a 0.5M 9-BBN (0.41 mol) solution in hexane at 0-5°C over 3.5 hours. of the reaction mixture 11 B NMR spectrum shows a new broad singlet signal at d=-0.5, assigned to 9-BBN-5-ethyl-2-picoline complex (IR spectrum in hexane: BH stretching vibration 2300- 2400cm -1 ). The solvent was distilled off under vacuum from half of the prepared hexane solution to leave an amber pyrophoric liquid, 47.5 g (95% yield). 11 The BNMR spectrum shows a broad singlet at d = -1.6 (95% purity) assigned to the product.
Embodiment 3
[0070] Example 3: Preparation of bis(2,5-dimethylhex-4-en-3-yl)borane-2-picoline complex in THF:
[0071] 2,5-Dimethyl-2,4-hexadiene (4.64g, 40mmol) was added to borane-tetrahydrofuran complex (20ml, 1M, 20mmol BH 3 )middle. After hydroboration was complete, 2-picoline (1.83 g, 20 mmol) was added to the solution of bis(2,5-dimethylhex-4-en-3-yl)borane. The bis(2,5-dimethylhex-4-en-3-yl)borane-2-picoline complex is shown at d = -3.2 11 B NMR signal (broad singlet, 85% purity).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com