Medicinal composition containing levodopa and benserazide hydrochloride
A technology of benserazide hydrochloride and levodopa, which is applied to the pharmaceutical composition of symptomatic Parkinson's syndrome, and the field of treating Parkinson's disease, can solve the problem of color change, benserazide hydrochloride light and wet instability, and difficult finished product quality. control issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
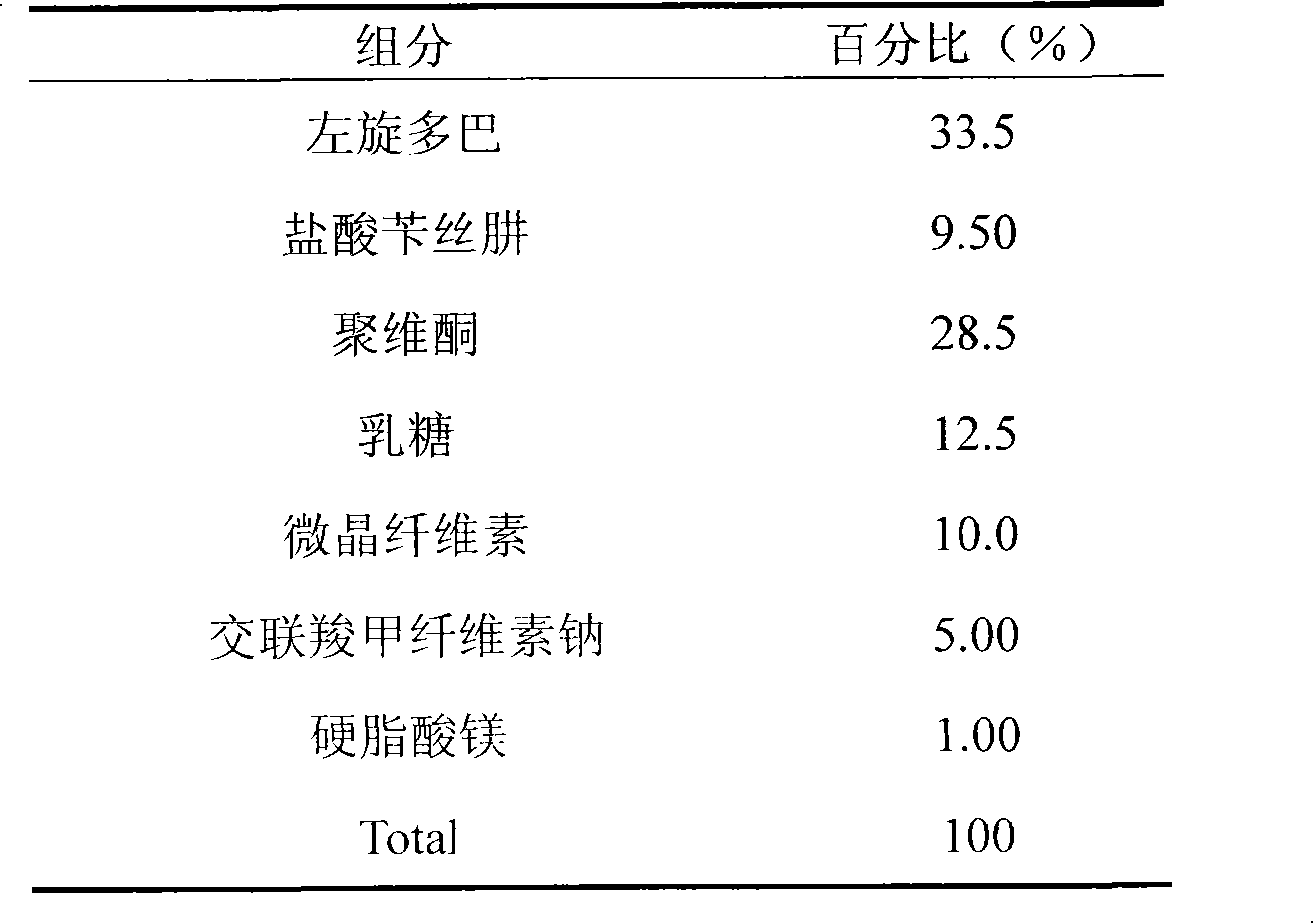
Embodiment 1
[0016]
[0017] Preparation:
[0018] The prescription amount of benserazide hydrochloride is weighed, placed in the ethanol solution of povidone, and spray-dried to obtain the carrier substance of benserazide hydrochloride. Mix the carrier substance of benserazide hydrochloride and other auxiliary materials evenly, put it into a tablet machine, and press it into tablets to obtain the product.
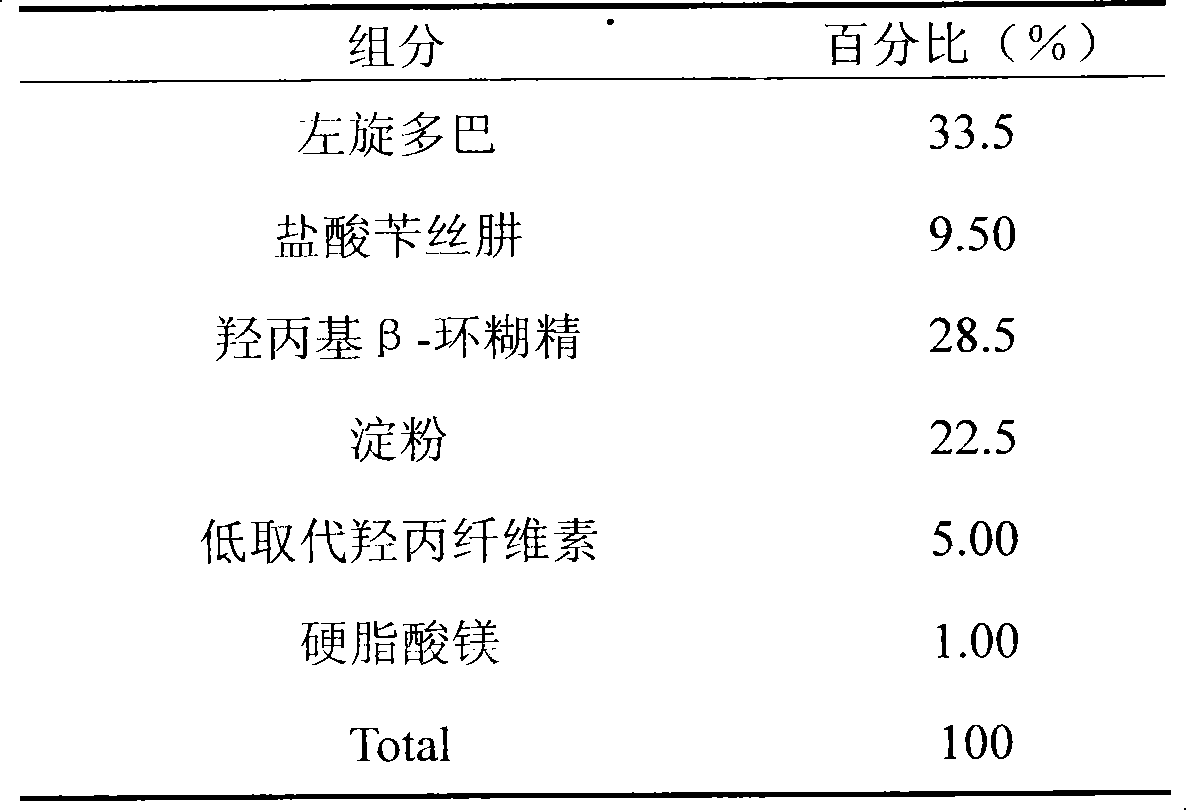
Embodiment 2
[0020]
[0021] Preparation:
[0022] Weighing a prescribed amount of benserazide hydrochloride, placing it in an aqueous solution of hydroxypropyl β-cyclodextrin, and spray-drying to obtain a carrier substance of benserazide hydrochloride. Mix the carrier substance of benserazide hydrochloride and other auxiliary materials evenly, put it into a tablet machine, and press it into tablets to obtain the product.
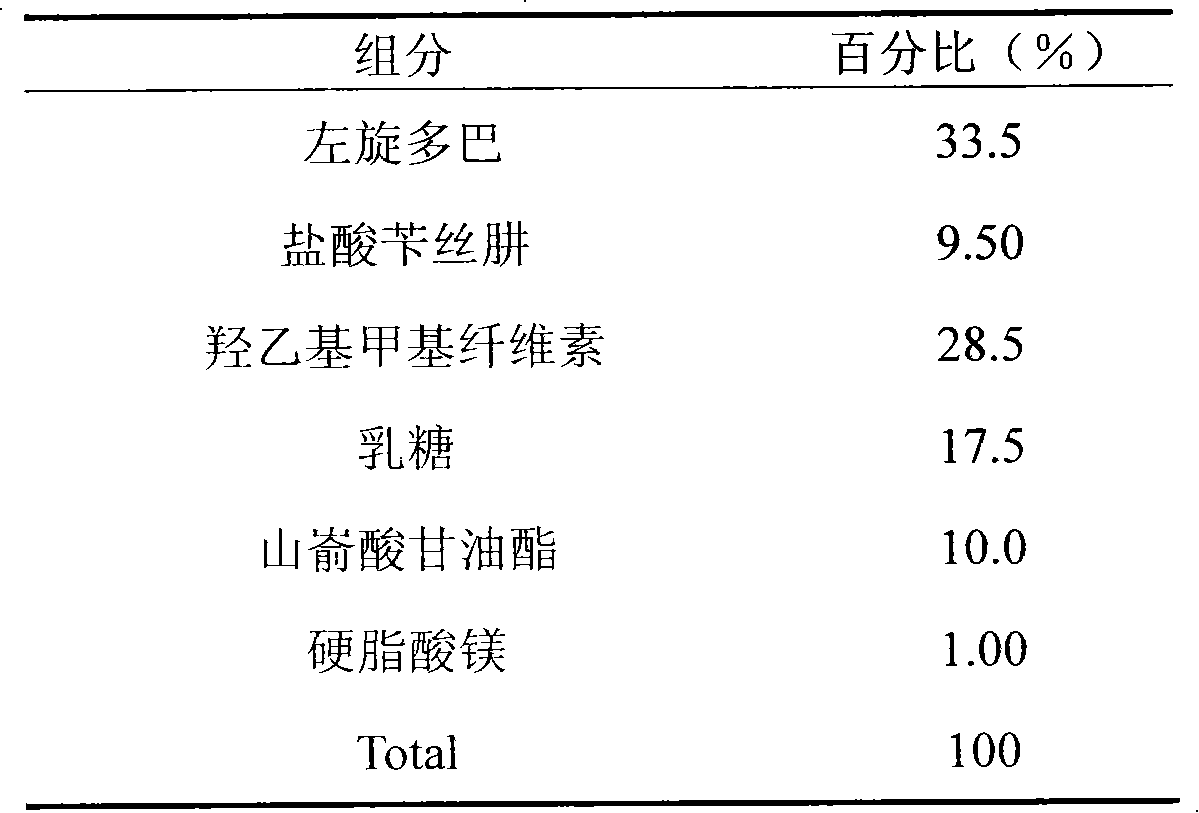
Embodiment 3
[0024]
[0025] Preparation:
[0026] Weighing the prescribed amount of benserazide hydrochloride, placing it in ethanol solution of hydroxyethyl methylcellulose, and spray drying to obtain the carrier substance of benserazide hydrochloride. Mix the carrier substance of benserazide hydrochloride and other auxiliary materials evenly, put it into a tablet machine, and press it into tablets to obtain the product.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap