Compound medicine for treating Parkinson's disease and preparation method thereof
A Parkinson's disease and compound technology, which is applied in the field of compound drugs for the treatment of Parkinson's disease and its preparation, can solve the problems of Parkinson's patients who have a certain risk of swallowing, orally disintegrating tablets are not on the market, and the preparation process is cumbersome. The effect of treatment cost, saving resources, and saving process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
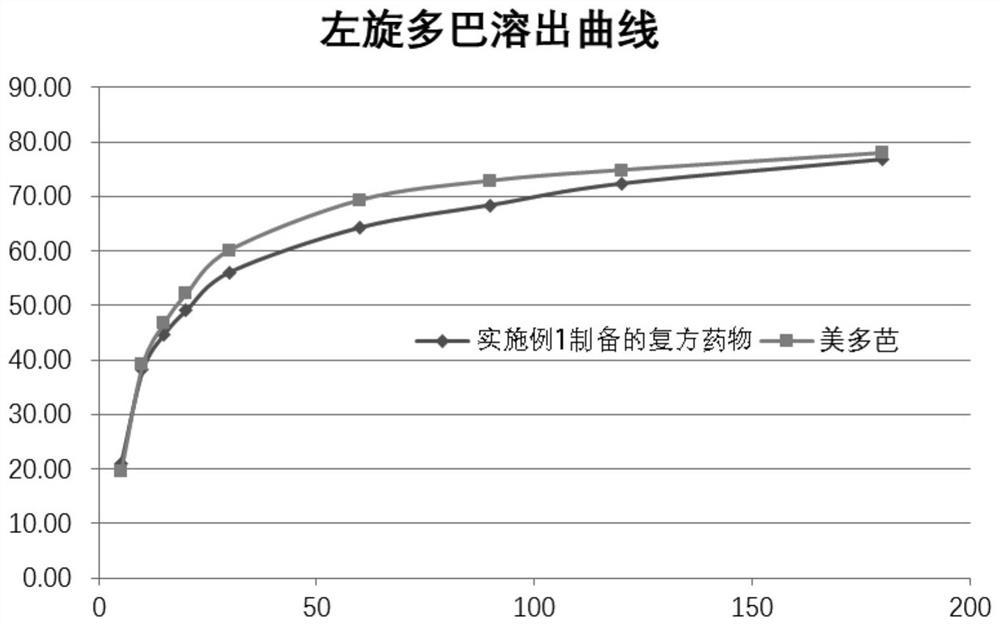
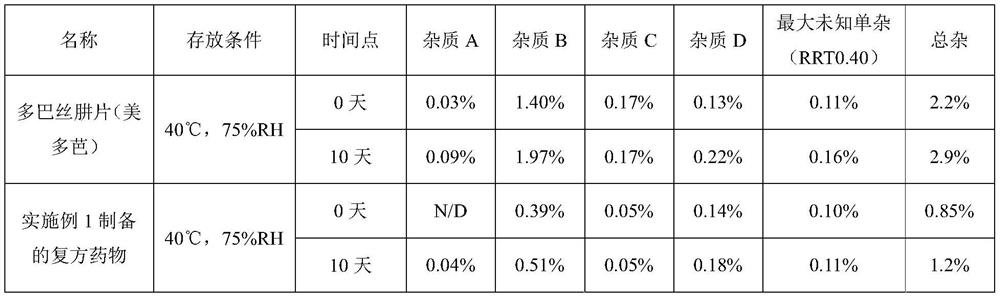
Image
Examples
Embodiment 1
[0056] Embodiment 1 compound medicine for treating Parkinson's disease and preparation method thereof
[0057] The present embodiment provides a compound drug for the treatment of Parkinson's disease, which is a tablet, and the content of every 1000 tablets is as follows:
[0058] Element Content (g) Features Levodopa 250 main ingredient Benserazide Hydrochloride 56 main ingredient microcrystalline cellulose 4.95 disintegrant Mannitol 50 filler Calcium hydrogen phosphate 50 fillers, blockers pregelatinized starch 18.7 Adhesive Crospovidone 12 disintegrant povidone 11 Adhesive Ethyl cellulose 5 protective coating Magnesium stearate 5.5 lubricant
[0059] The preparation method of this compound medicine is as follows:
[0060] Separately pulverize levodopa, benserazide hydrochloride and various auxiliary materials, then sieve, and save for future use.
[0061] The preparation metho...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap