A kind of continuous flow method for synthesizing benserazide hydrochloride
A technology of benserazide hydrochloride and benserazide hydrochloride, which is applied in the preparation of hydrazide, organic chemistry, preparation of hydrazone, etc., can solve the problems of unavoidable excessive reaction impurities, which is not conducive to improving production capacity, etc. The effect of industrialized production and low operating costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
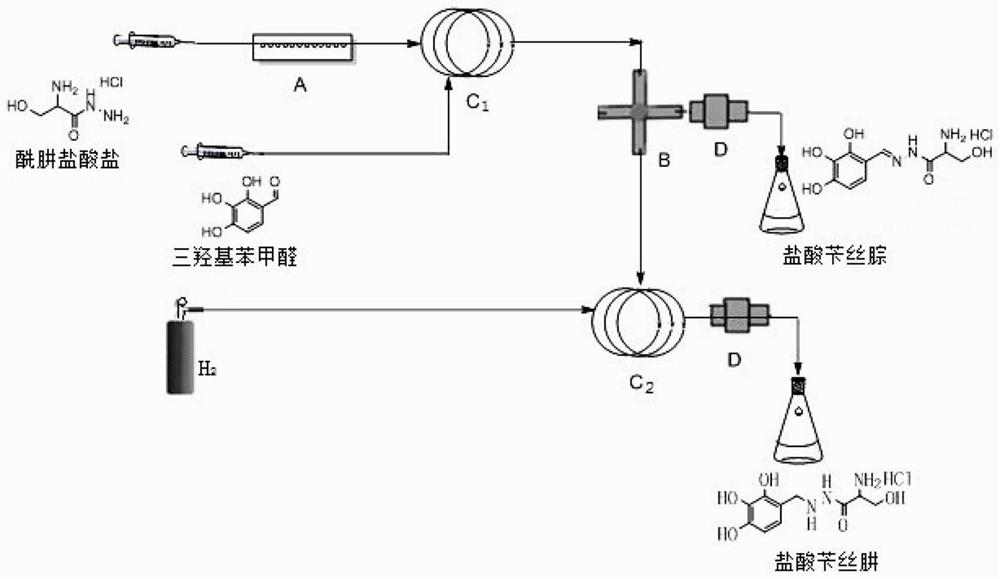
[0038] refer to figure 1 Flow chart of continuous flow synthesis of benserazide hydrochloride, figure 1 Among them, A is a heat exchanger, B is a flow divider, and the intermediate can be separated by valve adjustment or directly proceed to the next step of reaction: C is a tubular reactor, including a mixer, a horizontal tubular reactor, a vertical tubular reactor, Coiled tube reactors, U-shaped tube reactors, multi-tube parallel tube reactors, etc., D is a back pressure valve, which can automatically adjust the system pressure in real time, trace the process conditions of the flow chemical process, and then achieve reproducible reactions sex.
[0039] A method for continuous flow synthesis of benserazide hydrochloride, the method comprises the following steps:
[0040] S1: Pump 2mol / L ethanol solution of hydrazide hydrochloride (starting material) into heat exchanger A with a residence time of 0.5s, and then transport it to the first tubular reactor C at a flow rate of 10m...
Embodiment 2
[0046] S1: Pump 2mol / L ethanol solution of hydrazide hydrochloride (starting material) into heat exchanger A with a residence time of 0.5s, and then flow into the first tubular reactor C at a flow rate of 12ml / min 1 , the ethanol solution of the trihydroxybenzaldehyde of 2mol / L flows into the first tubular reactor C according to the flow velocity of 12ml / min 1 , react to obtain benzsilhydrazone hydrochloride;
[0047] S2: The benzsilhydrazone hydrochloride ethanol suspension obtained in S1 flows into the second tubular reactor C filled with palladium carbon catalyst 2 , the total flow rate is 30ml / min, and the residence time of 20s is maintained, while the second tubular reactor C 2 Into the hydrogen, to ensure that the second tubular reactor C 2 The internal pressure is 0.1Mpa;
[0048] S3: After continuously collecting the reaction solution, the organic phase is concentrated under reduced pressure, and ethanol is recovered to obtain crude product benserazide hydrochloride...
Embodiment 3
[0051] S1: Pump 2mol / L ethanol solution of hydrazide hydrochloride (starting material) into heat exchanger A with a residence time of 0.5s, then flow into the first tubular reactor C at 13ml / min 1 , the ethanolic solution of trihydroxybenzaldehyde with a concentration of 2mol / L flows into the first tubular reactor C according to the flow velocity of 13ml / min 1, the reaction obtains the ethanol solution of benzylhydrazone hydrochloride;
[0052] S2: The benzsilhydrazone hydrochloride ethanol suspension obtained in S1 flows into the second tubular reactor C filled with palladium carbon catalyst 2 , with a total flow rate of 40ml / min, maintaining a residence time of 20, while feeding the second tubular reactor C 2 Into the hydrogen, to ensure that the second tubular reactor C 2 The internal pressure is about 1.0Mpa;
[0053] S3: After continuously collecting the reaction solution, the organic phase is concentrated under reduced pressure, and ethanol is recovered to obtain crud...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap