New application of asperosaponin VI
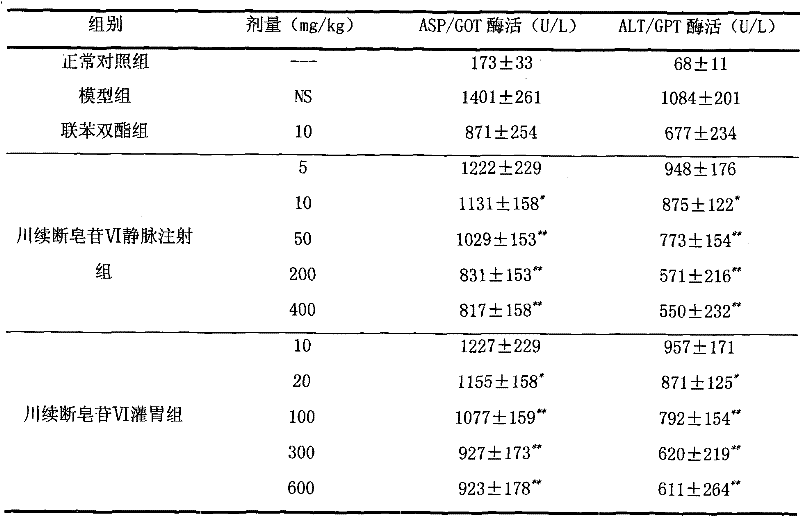
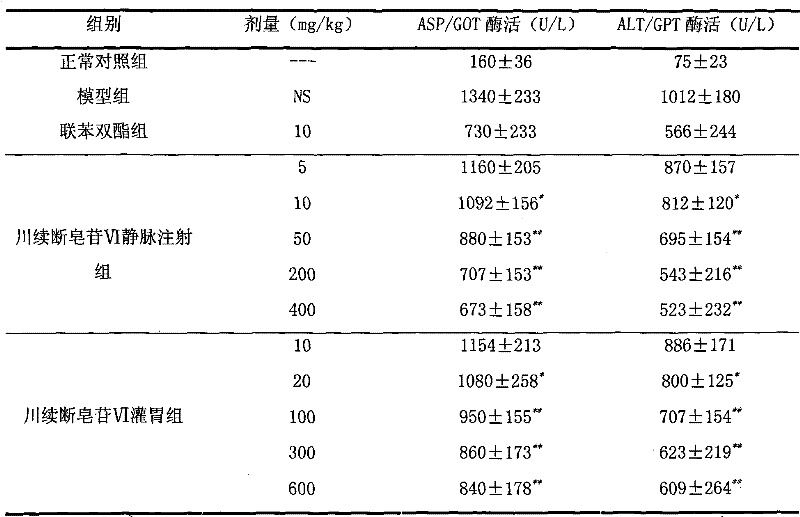
Dipsacus saponin, the only technology used in the field of drugs for the treatment of liver diseases, can solve the problems of high cost, easy recurrence, unsatisfactory treatment effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
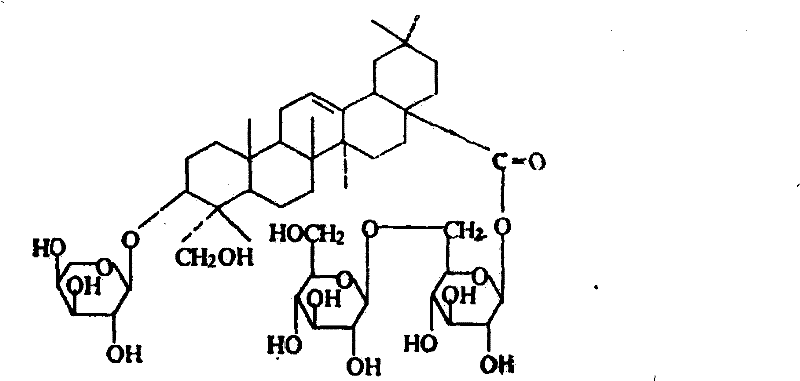
[0016] Preparation Example 1: Preparation of Dipsacus saponin VI
[0017] Take 1 kg of Dipsacus chuanxiong medicinal material, crush it, pass through a 10-mesh sieve, add 10 times the amount (V / W) of 70% ethanol to soak overnight, reflux extraction twice, each time for 2 hours, and recover the ethanol from the extract until it has no alcohol smell. Add ethanol to the concentrated solution until the alcohol concentration is 80%, and let stand overnight; filter with suction, concentrate the filtrate to no alcohol smell, add 5 times the amount of water, let stand overnight, filter, and treat the AB-8 macroporous resin on the filtrate (resin The weight ratio of medicinal materials is 1:1), wash 2 column volumes with 0.5% sodium hydroxide solution, wash with water to neutrality, then elute with 40% ethanol for 3 column volumes, discard, and finally wash 3 column volumes with 50% ethanol Column volume, collect 50% ethanol eluent, concentrate and evaporate to dryness to obtain about ...
preparation example 2
[0018] Preparation Example 2: Preparation of Dipsacus saponin VI
[0019] Take 1 kg of Dipsacus chuanxiu medicinal material, crush it, pass through a 10-mesh sieve, add 8 times the amount (V / W) of 75% ethanol to soak overnight, reflux extraction three times, each time for 2 hours, recover the ethanol from the extract until it has no alcohol smell, filter , the processed D101 macroporous resin on the filtrate (resin and medical material weight ratio 1: 1), 1.0% sodium hydroxide solution washes 2 column volumes and washes with water to neutrality, then uses 35% ethanol to elute 3 column volumes , discarded, and finally washed 3 column volumes with 50% ethanol, collected 50% ethanol eluate, concentrated and evaporated to dryness to obtain about 60g of crude extract (the content of Dipsacus saponin VI is about 60%); The material was put on a silica gel column, and eluted with chloroform-methanol (2:1) as a solvent, and the eluate was collected, concentrated, and crystallized repea...
preparation example 3
[0020] Preparation Example 3: Preparation of Chuanxuan Saponin VI Injection:
[0021] prescription:
[0022] Dipsacus saponin VI 5.0g
[0023] Sodium chloride 89g
[0024] Water for injection 10000ml
[0025] Made into 10000ml
[0026] Preparation method:
[0027] Measure Dipsacus saponin VI 5.0g and sodium chloride 89g according to the prescription, dissolve them in 10,000ml of water for injection, stir, add 0.2% activated carbon, stir for 20 minutes, filter the solution through a microporous membrane to clarify, and pack in 125ml infusion bottles Medium, 100mL per bottle, sterilized and packed after passing the inspection. Other inspection items should meet the requirements for injection items in the Pharmacopoeia of the People's Republic of China 2005 Edition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com