Production of bivalve tetraploid molluscs from diploid parents
A mollusk and tetraploid technology, applied in animal cells, climate change adaptation, animal husbandry, etc., can solve the problems of difficult genetic improvement, low competitiveness of tetraploid larvae, and heavy load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Induction of polyploidy and reproduction of larvae
[0045] The diploid parents used for the experiments were from the natural wild-type diploid oyster egg collection area in the French oyster Oléron Bay (Marennes Oléron Bay). Six female parents and four male parents were used for aggregate pairing by gonad scarification method. Fifteen million oocytes and 3 billion motile sperm from 10 parents were used for hybridization in 1 liter of filtered seawater at 25°C. The maintenance of the first polar body was affected by using cytokinostatin B (CB) dissolved in DMSO to a final concentration of 0.5 mg / L. From the 10th minute after fertilization, CB was used for 15 minutes.
[0046] This protocol for inducing polyploidy is similar to those already disclosed in the literature for the production of triploids (GUO et al., Biol. Bull., 183, 381-93, 1992; et al., Aquaculture, 174, 229-421999). However, the final concentration of CB was lowered (0.5 mg / L ...
Embodiment 2
[0089] Example 2: Settlement and growth of tetraploid oyster eggs
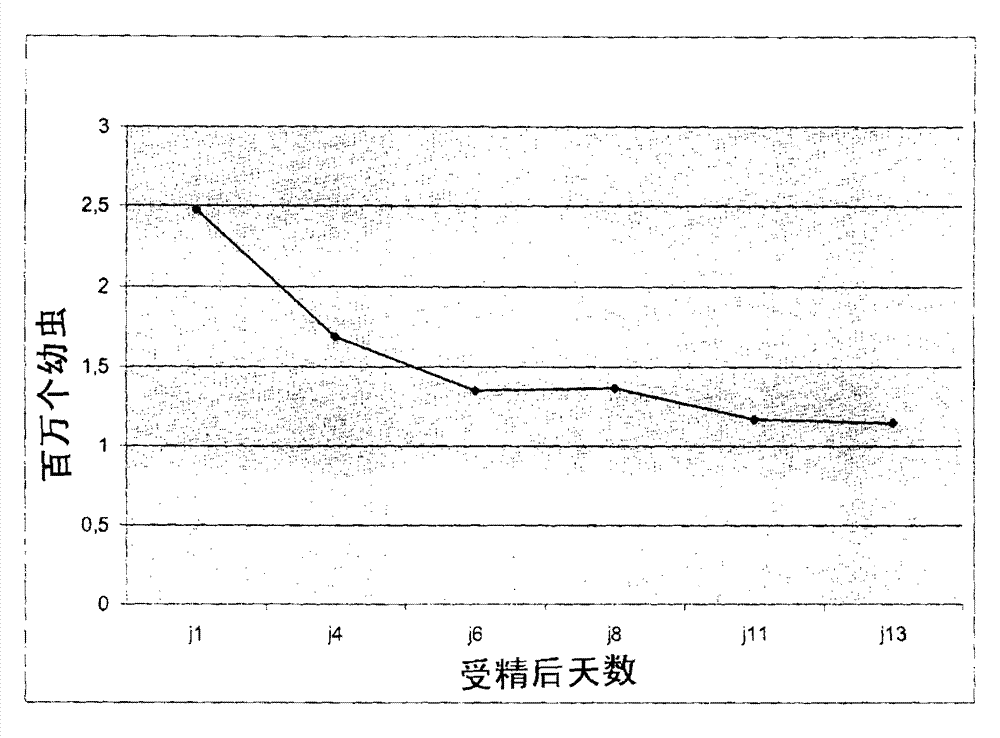
[0090] 1 month after settling
[0091] One month after establishment, checks for the presence of tetraploid oyster eggs were performed by flow cytometry within successfully denatured and established larval populations. Due to the small size of oyster roe at this stage, this operation was sometimes performed destructively on samples containing some oyster roe. Put the oyster roe into a 1.5mL test tube. They were then incubated in 1 mL of lysis buffer, and they were triturated by using a plastic stopper. Finally, the released nuclei were filtered through a 30 μm mesh nylon filter (Partec Celltrics) and collected in cytometry assay tubes, labeled with DAPI and analyzed as described in Example 1 above.
[0092] Cell count results allowed us to confirm that, in addition to triploid and diploid populations, tetraploid oyster egg (29%) populations were also significantly present ( Figure 30 Mi...
Embodiment 3
[0115] Example 3: Production of tetraploids in mussels
[0116] By using the methods detailed in Examples 1 and 2, tetraploidy was efficiently obtained in mussels (M. edulis and Mytilus galloprovincialis).
[0117] In mussels, cell count results confirmed the presence of a predominant tetraploid oyster egg population in addition to the triploid and diploid populations. therefore,
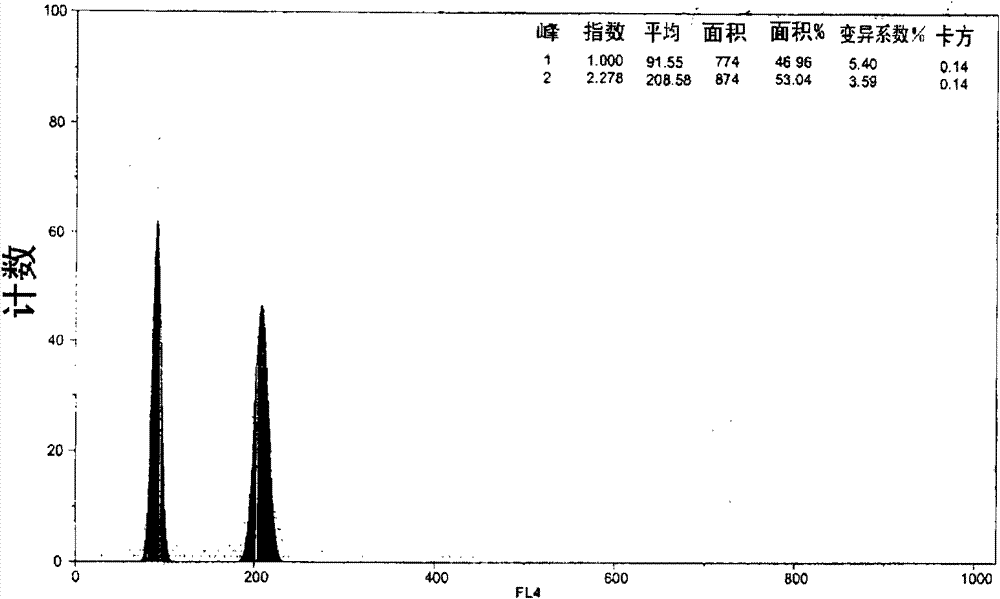
[0118] - Control hybridization can provide an exclusive population of diploid nuclei ( Figure 37 , peak "RN1");
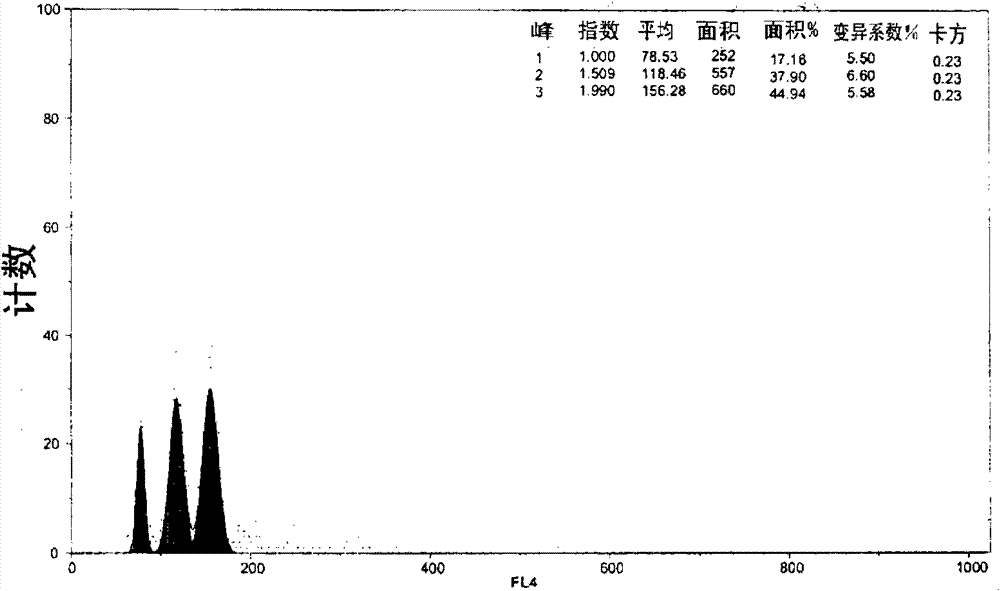
[0119] - Induced samples maintained by GPI showed three types of nuclei populations ( Figure 38 ), corresponding to the following ploidy levels: diploid (average around 10%, peak "RN1"), triploid (average around 20%, peak "RN2") and tetraploid (average around 70%, peak "RN2") peak "RN3"). The resulting tetraploid individuals are in the process of breeding.
[0120] In summary, by using the methods detailed in the examples above, viable tetraploids can be produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com