Inhibitor of fatty acid synthase and application thereof
A technology of fatty acid synthase and inhibitors, which is applied in the field of fatty acid synthase inhibitors, can solve the problems of limited application value and insufficient inhibitory activity, and achieve the effect of great application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1, the preparation of fatty acid synthase inhibitor
[0048] Wash the tea bar maple leaves collected in September in Jingbo Lake, Heilongjiang Province, dry them naturally or dry them at low temperature (<50°C) and pulverize them, and prepare fatty acid synthase inhibitors by the following method:
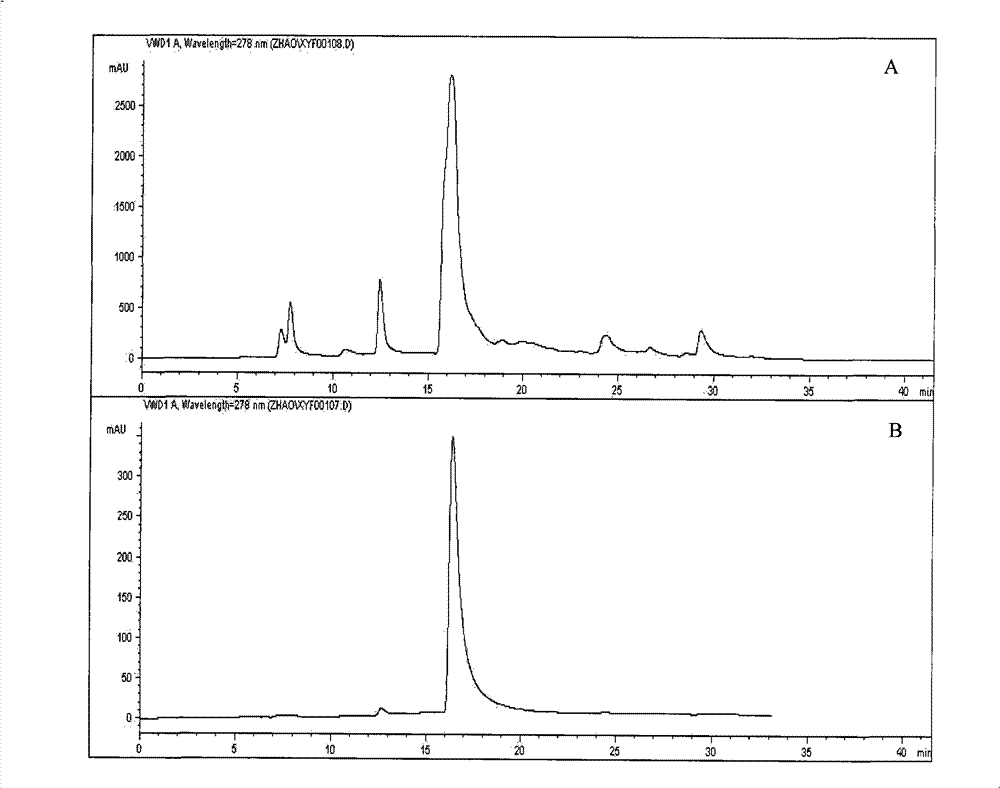
[0049] Use ethanol-water mixed solvent (the volume percentage of ethanol is 75%) to mix with the above-mentioned pulverized tea bar maple leaves in a mass and fraction ratio of 8:1, reflux at 78° C. for 1.5 hours, and filter; repeat the above-mentioned reflux operation for two times under the same conditions. times, filtered, and the three filtrates were combined. Evaporate and concentrate under reduced pressure and low temperature (0.09MPa, 55°C) to remove ethanol to obtain an aqueous phase containing effective substances, dissolve it with an appropriate amount of methanol, and then extract with an equal volume of petroleum ether (boiling point 60-90°C) for 3-4 t...
Embodiment 2
[0124] Embodiment 2, the detection of FAS inhibitory activity of fatty acid synthase inhibitor of the present invention
[0125] NADPH, acetyl-CoA, malonyl-CoA, and DTT (dithiothreitol) used in the test were all purchased from Roche Diagnostics.
[0126] At 37°C, add FAS substrates to a 2ml quartz cuvette: 25μl of 0.2mM acetyl-CoA solution, 50μl of 0.4mM malonyl-CoA solution and 50μl of 1.3mM NADPH solution, then add 0.1M pH7. Mix 1.85ml of 0 phosphate buffer solution and 25μl of FAS solution to start the enzymatic reaction. The activity of FAS was expressed by detecting the change (ΔA / min) of the absorbance value A at a wavelength of 340nm per unit time with a spectrophotometer. In the presence of the fatty acid synthase inhibitor solution prepared in a certain concentration embodiment 1, the activity of the FAS holoenzyme reaction decreases, and the concentration of the required fatty acid synthase inhibitor when its activity drops by half is IC 50 , using this value to re...
Embodiment 3、 5
[0134] Example 3, Detection of FAS Inhibitory Activities of Pentagalloyl Gluconic Acid, Methyl Gallate and Drobetin-3-O-L-Rhamnoside
[0135] The specific determination method is the same as the detection of the inhibitory activity of fatty acid synthase inhibitors on FAS in Example 2.
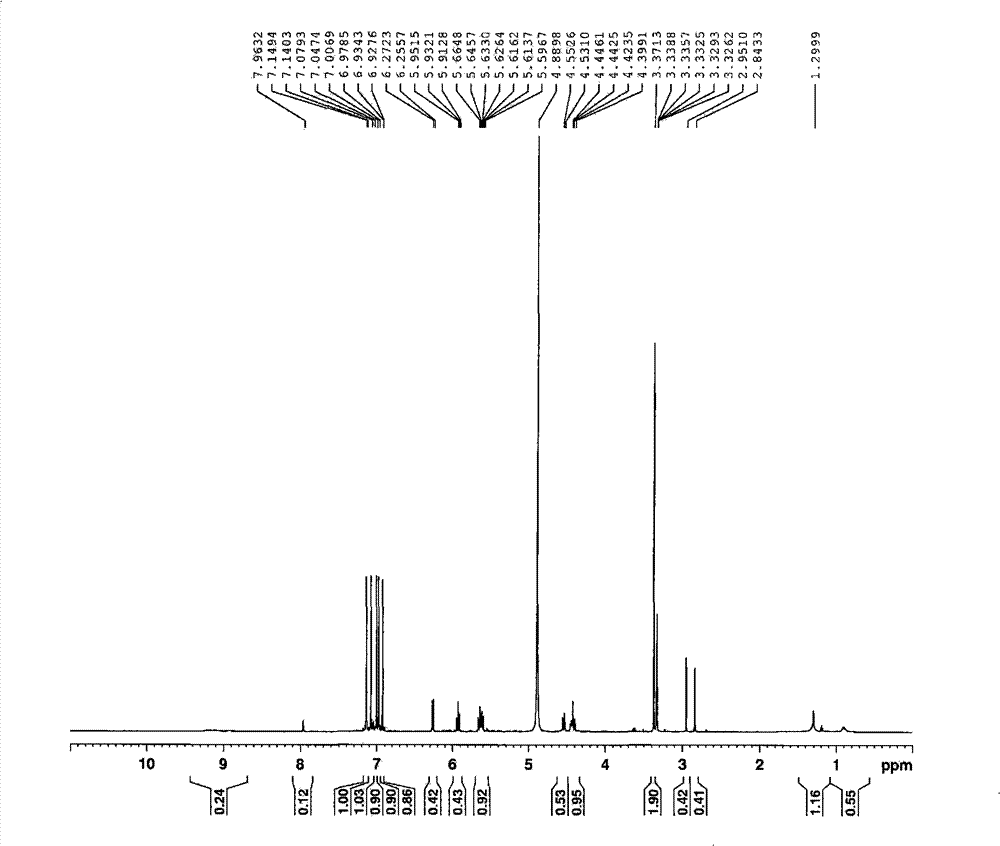
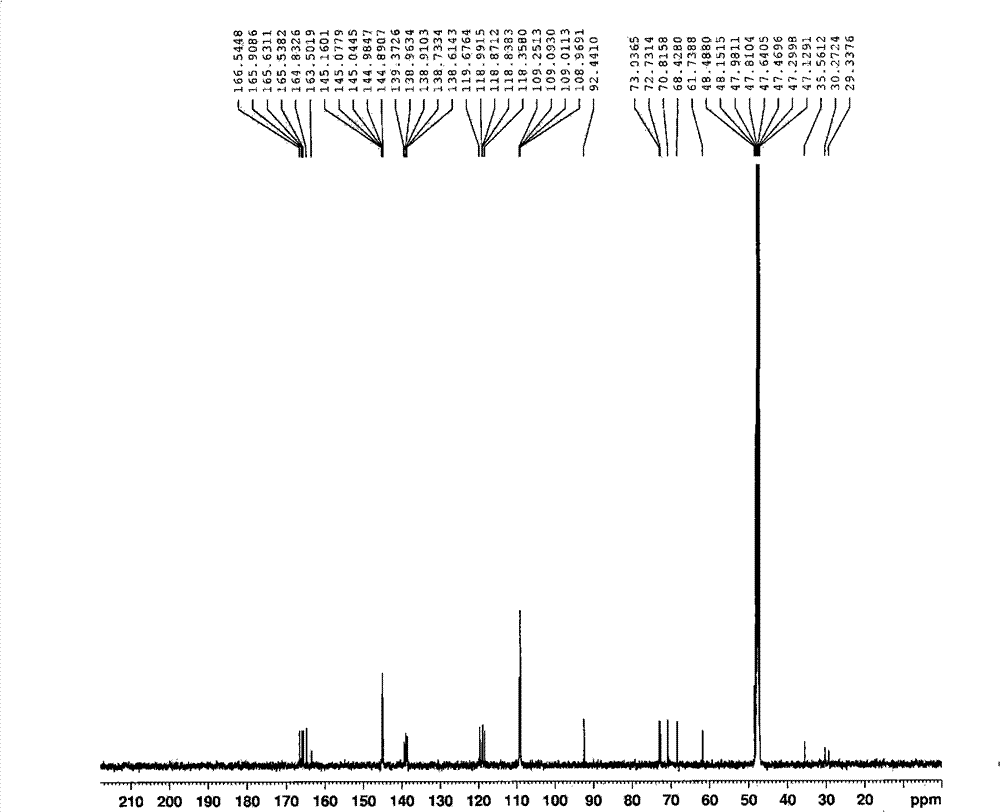
[0136] Preparation of pentagalloyl gluconic acid solution: 16.8 mg of pentagalloyl gluconic acid isolated in Example 1 was dissolved in 1 ml of methanol to obtain a pentagalloyl gluconic acid solution with a concentration of 16.8 mg / ml.
[0137] Preparation of methyl gallate solution: 16 mg of the methyl gallate isolated in Example 1 was dissolved in 1 ml of methanol to obtain a methyl gallate solution with a concentration of 16 mg / ml.
[0138] Preparation of the dendrobetin-3-O-L-rhamnoside solution: 20 mg of the dendrobetin-3-O-L-rhamnoside isolated in Example 1 was dissolved in 1 ml of methanol to obtain dendrobite with a concentration of 20 mg / ml Prime-3-O-L-rhamnoside solution.
[0139]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com