Local administration of chicken yolk immune globulins (igy) to treat and prevent fungal infections
A technology of fungi, Candida albicans, applied in the diagnosis of fungi, drugs for the prevention and/or treatment of all types of fungal infections, and the prevention or treatment of such diseases, which can solve the problems of loss of activity and unsuitability for long-term use in humans
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Preparation of fungi
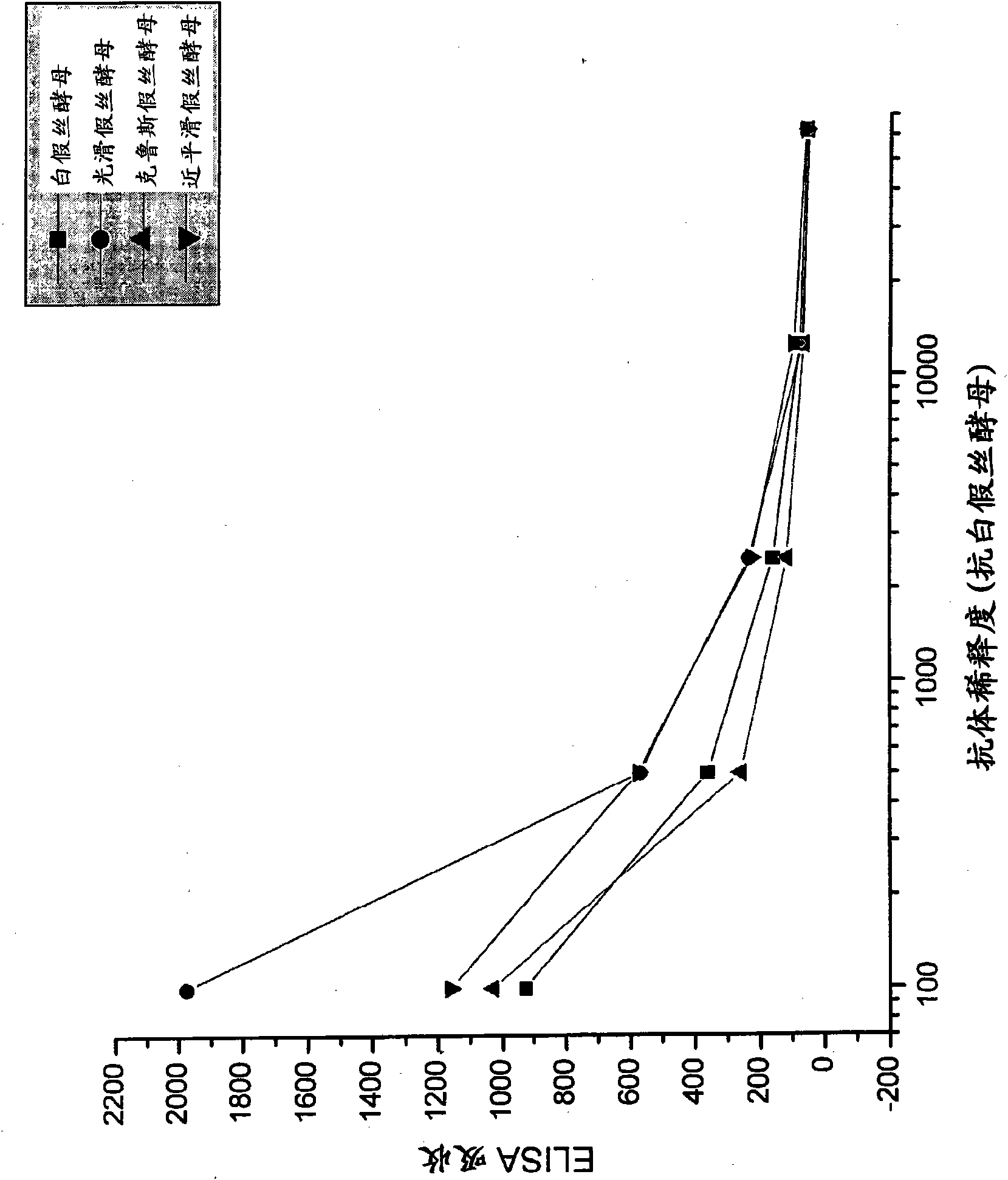
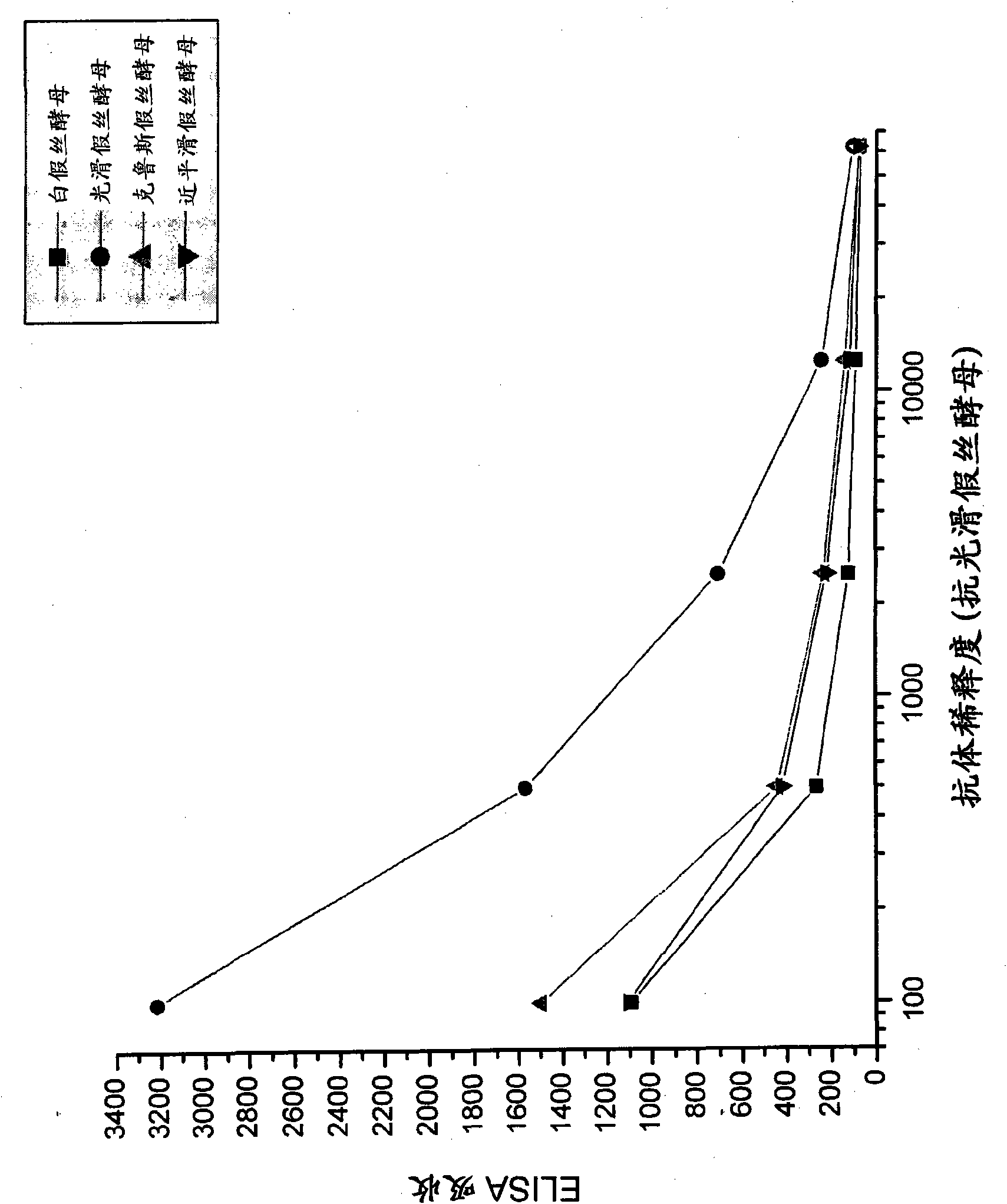
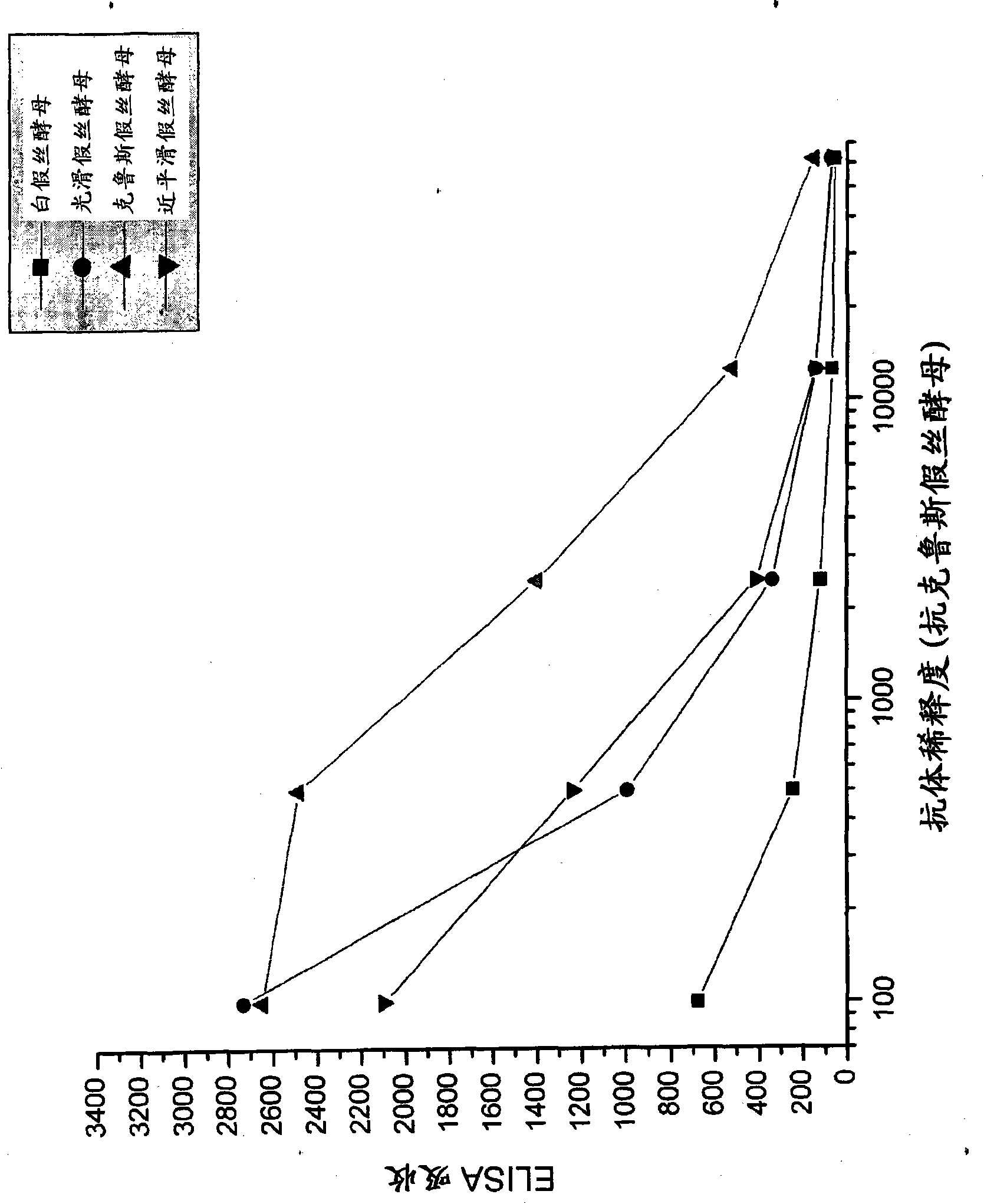
[0092] Candida species isolated from infected patients were used in in vitro experiments to demonstrate the preventive potential of egg immunoglobulins isolated from chickens hyperimmunized with fungal antigens.
[0093] The fungi were cultured in 500 ml culture flasks containing 100 ml of 2% glucose supplemented with amino acids, 0.15% yeast nitrogen source, and 0.5% ammonium sulfate. The flasks were shaken at 200 rpm at 37°C for 24 hours in a rotary incubator. The fungi were also cultured on Candida plates used to detect Candida colonization in patient samples.
[0094] Preparation of anti-Candida IgY immunoglobulin
[0095] Formaldehyde-killed Candida albicans washed in saline, Candida glabrata , Candida krusei mother and Candida parapsilosis suspension and freeze. Separate groups of chickens were immunized with each Candida species. Use 10 per chicken per immunization 7 Candida. White Leghorn chickens were immunized intramuscular...
Embodiment 2
[0098] Cell Adhesion Assay
[0099] Adhesion to the mucosal epithelium is considered the first stage of infection. In this example, epithelial cells and Pseudomonas aeruginosa (as a representative of bacteria) were used to evaluate adhesion.
[0100] Fresh cells cultured for 24 hours were washed by centrifugation in PBS and resuspended in PBS with IgY from eggs immunized with Pseudomonas or IgY from non-immunized chickens (100:1 ratio). Alternatively mix with medium without IgY at all and incubate at 37°C for 2 hr. After incubation, the culture was filtered through a 45 gm filter to remove any non-adherent cells, washed and resuspended in PBS.
[0101] Adhesion was assessed by microscopy at 400X, using a graded micrometric grid to aid in accurate counting. The adhesion rate is expressed as the percentage of cells visibly adhered to Pseudomonas aeruginosa.
[0102] The results showed that the adhesion of Pseudomonas aeruginosa to epithelial cells was reduced by more than 50...
Embodiment 3
[0111] Example 3: Freeze-drying and concentration of IgY extracts
[0112] double freeze drying
[0113] 20 freeze-dried vials each containing 4 ml of the IgY extract of the present invention were provided. 8.5 mg of lactose was added to 10 of the bottles. 34 mg of lactose was added to the remaining 10 vials. Vials were freeze-dried according to standard protocols.
[0114] After drying, two vials of each lactose concentration were set aside and recorded as "single freeze-dried". The product in the remaining bottle was dissolved in distilled water according to the table below. For each lactose concentration ("high" or "low"):
[0115] Add 4ml of water to two of the bottles
[0116] Add 2ml of water to two of the bottles
[0117] Add 1ml of water to two of the bottles
[0118] Add 0.5ml of water to two of the bottles
[0119] The amount of water added was marked on the bottle (ie 4, 2, 1 and 0.5 respectively). Sixteen vials containing reconstituted IgY were lyophili...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com