Fluorescent quantitative PCR (Polymerase Chain Reaction) detection kit and detection method of HBV (Hepatitis B Virus) and TP (Treponema Pallidum) of donor corneas
A technology for detection kits and kits, which can be used in the determination/testing of microorganisms, biochemical equipment and methods, fluorescence/phosphorescence, etc., and can solve problems such as low sensitivity, PCR false positive pollution, and difficulty in improving quantitative accuracy. High quantitative accuracy and the effect of avoiding cross-contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
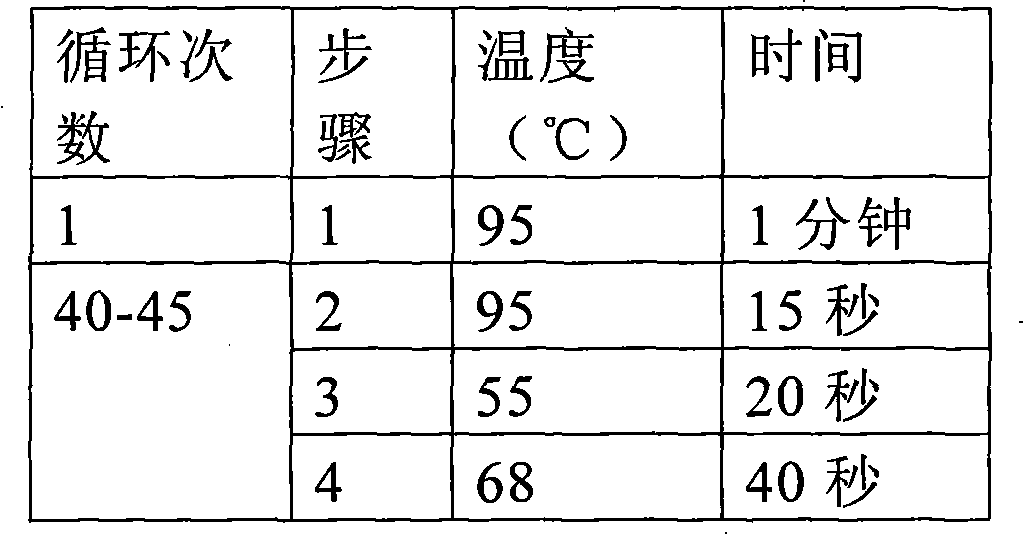
[0046] Example 1 Donor corneal HBV virus detection:
[0047] 1. DNA extraction from donor eyeball conjunctival tissue and construction of positive working standards:
[0048] (1) Take 5 mg of the conjunctival tissue of the donor eyeball, put the tissue block into a centrifuge tube and cut it into pieces, and add 180 μL of tissue lysate. Add 20μL proteinase K, mix well, and incubate at 56℃ until the tissue is completely lysed;
[0049] (2) Add 200μL of purified lysate, vortex the shaker for 15s to mix, and incubate at 70°C for 10min. Add 200μL of absolute ethanol to the sample, vortex the shaker for 15s to mix;
[0050] (3) Transfer the sample from step 2 to the filter column, close the lid and centrifuge at 8000 rpm for 1 min. Discard the waste liquid in the filter column, add 500 μL of eluent 1, and centrifuge at 8000 rpm for 1 min;
[0051] (4) Discard the waste liquid in the filter column, add 500 μL of eluent 2, and centrifuge at 12000 rpm for 3 min. Discard the waste liquid in ...
Embodiment 2
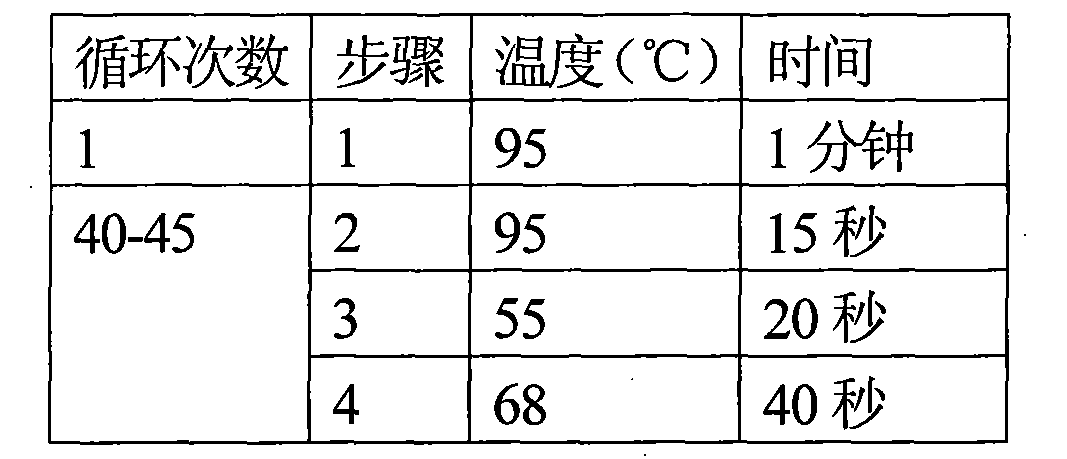
[0066] Example 2 TP detection of donor cornea:
[0067] 1. Extraction of DNA from donor eyeball conjunctival tissue and construction of positive working standards: The specific steps are the same as the extraction of donor eyeball conjunctival tissue DNA in Example 1.
[0068] Construct a plasmid containing TP gene (pUC57-TP) as a positive working standard. The positive working standard contains the target sequence amplified by the TP forward and reverse primers. In addition, an extra sequence is added to each side of the target sequence to make it closer to the structure of the actual test sample to ensure the difference between the actual test sample Consistency of amplification efficiency.
[0069] Use the Promega PureYield plasmid miniprep system kit to extract the TP-positive working standard quality pellets, and then use an ultraviolet spectrophotometer to compare color at 260nm and 280nm to obtain the purity and concentration of the TP-positive working standard. According to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com