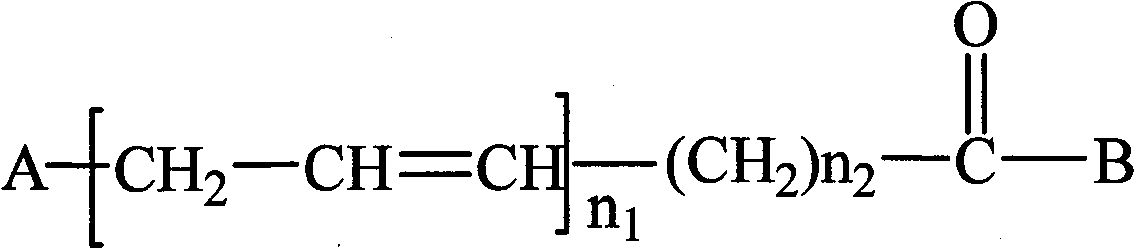Medicinal composition and application thereof
A kind of composition and medicine technology, applied in the pharmaceutical composition and its application field, can solve the problems such as the treatment effect is not ideal enough
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~31
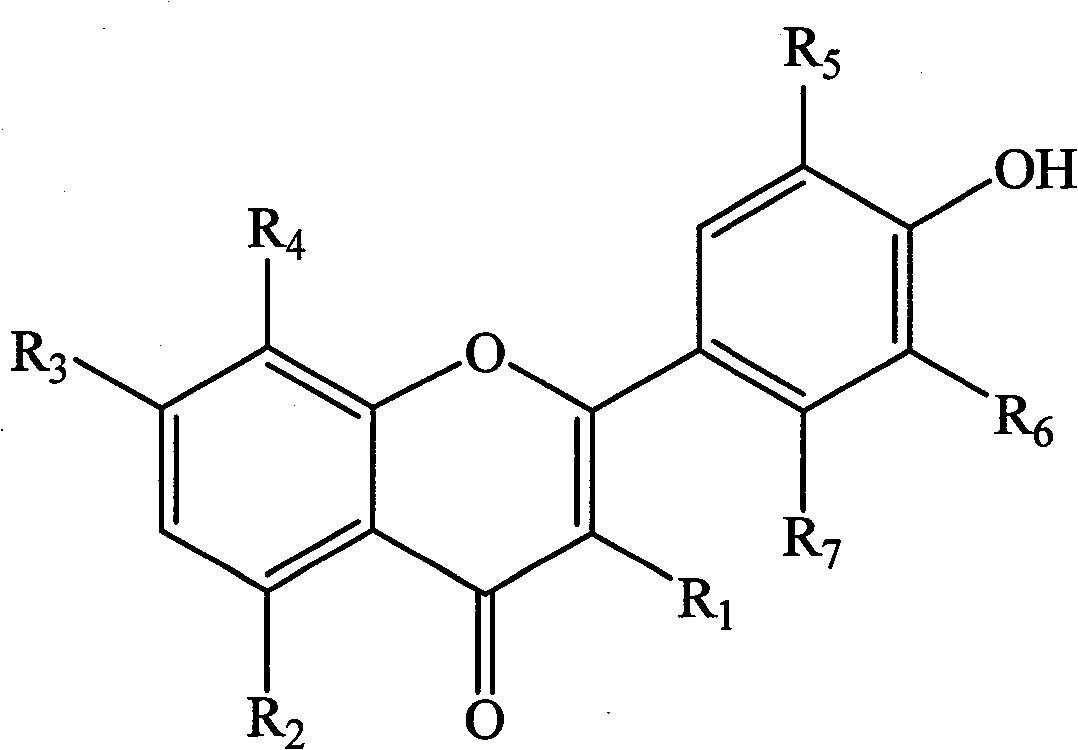
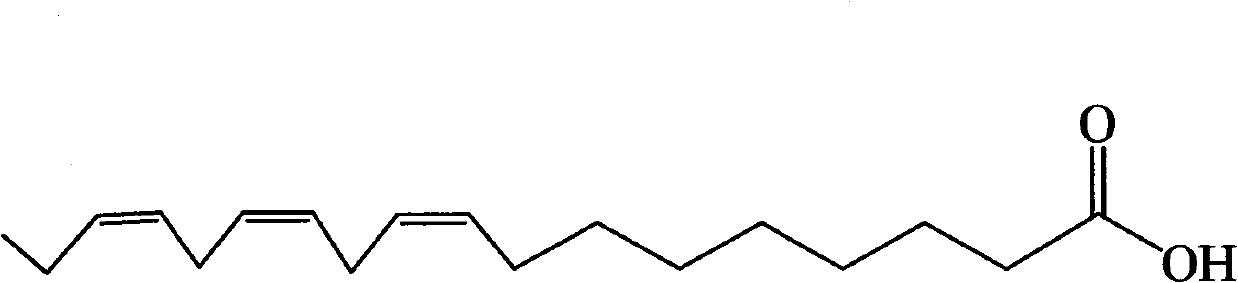
[0113] Linolenic acid, palmitic acid, N-(2-hydroxyethyl), 9,12,15-octadecatrienamide, glyceryl linolenate, 15 , 16-dihydroxylinoleic acid, ethyl linolenate, glyceryl palmitate, 24-methylenecholesteryl palmitate, 1,3-dipalmitic acid-2-linolenic acid triglyceride, 1,3- Dilinolenic acid-2-palmitic acid triglyceride, 24-methylene cholesteryl linolenate, cycloalkenyl eucalyptol linolenate, pollen alkanol sterol linoleate, 1,3-dipalmitic acid diglyceride, 1-palmitic acid-2-heptadecanoic acid diglyceride, kaempferol, quercetin, isorhamnetin, gossin, kaempferol 7-O-(6'-rhamnosyl) glucoside, N 1 , N 5 , N 10 -Three-(E)-coumaroylpermidine, N 1 , N 5 , N 10-Tris-(Z)-coumaroylperamidine, 1-O-(β-D-glucose)-(2S,3S,4R)-2N-[(2′R)-2′-Hydroxytetradecanoyl Acenoic acid]-octadecene-3,4-diol, putrescine and indole-3-acetic acid are mixed according to the components and contents described in Table 1 to obtain the pharmaceutical composition of the present invention.
[0114] Wherein, the pre...
Embodiment 29
[0194] Test product: Example 29: by mass percentage, the plant extract of fatty acid compounds is 45%, the proportioning of plant extract of flavonoid compounds is 35% and the plant extract of alkaloid compounds is 20%, and fatty acids, flavonoids are combined These three types of compounds of flavonoids and alkaloids obtain the anti-prostatic hyperplasia pharmaceutical composition of the present invention (wherein the active ingredients are about mass percent: 40% of fatty acid compounds, 30% of flavonoids and 10% of alkaloids) .
[0195] Positive control substance:
[0196] For Qianliekang (Pulean Tablets), the maximum clinical dosage for a 60 kg adult is 12 tablets / d.
[0197] Rat dose determination: According to the conversion relationship between human and animal effective doses, the rat dose is calculated as 6 times the clinical dose of a 60 kg adult, that is, 1.2 tablets / kg. Preparation method: Take 36 Qianliekang tablets, use distilled water as solvent, add Tween-80 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com