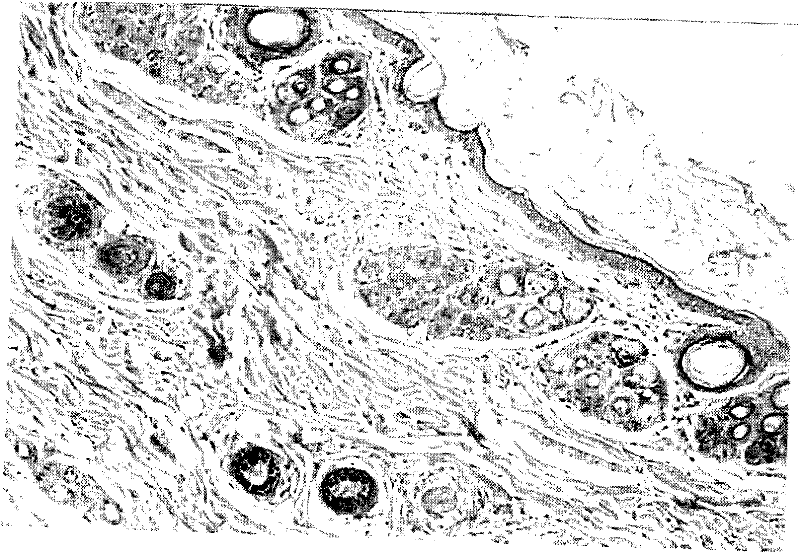
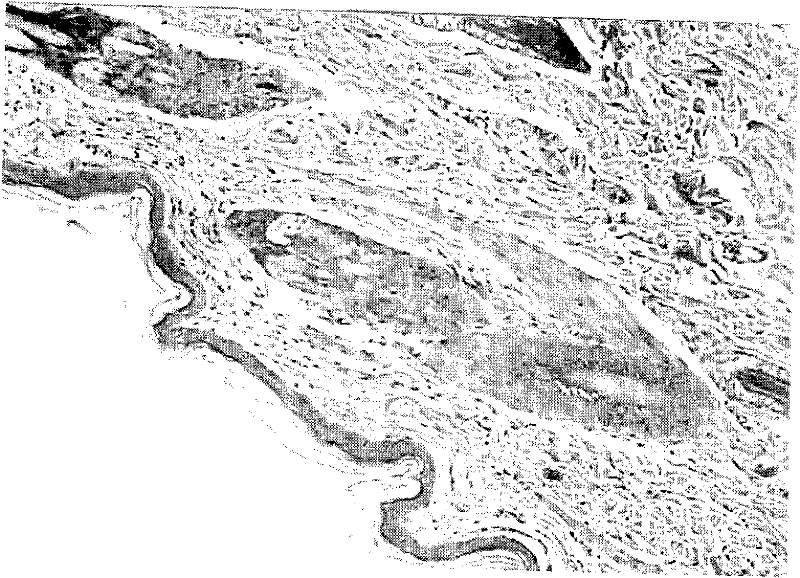
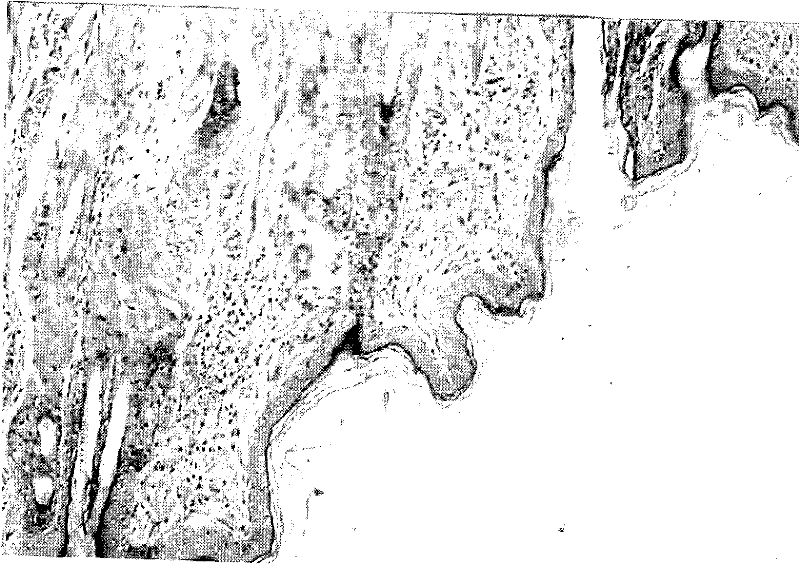
External medicinal composition for treating skin ulcer and preparation method thereof
A technology for external use of drugs and skin ulcers, which is applied in the field of medicine and can solve problems such as clinical application limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0161] The preparation of embodiment 1 refined white sugar
[0162] 1. The proportion of raw and auxiliary materials:
[0163] White sugar: deionized water: activated carbon: ethanol = 15:15:0.45:15 (mass ratio W / W)
[0164] 2. Preparation process
[0165] (1) Take by weighing 1500g of white sugar, 1500g of deionized water, 45g of activated carbon, 1500g of ethanol, and set aside;
[0166] (2) Put the weighed white sugar into the tank, add the weighed deionized water, stir and heat to dissolve at 80-85°C;
[0167] (3) Add the activated carbon that has been weighed, decolorize for 20-30 minutes, and filter while it is hot;
[0168] (4) concentrating under reduced pressure at 50-95° C. until the sugar content in the concentrated white sugar solution is 80% to 85%;
[0169] (5) When the temperature is lowered to 50-70°C, slowly add the weighed ethanol;
[0170] (6) Continue to cool down to 20-25°C, stir and crystallize for 3-4 hours;
[0171] (7) Centrifugal crystallized re...
Embodiment 2
[0174] The preparation of embodiment 2 compound refined white sugar ointment
[0175] 1. The proportion of raw and auxiliary materials:
[0176] Refined sugar: neomycin sulfate: citric acid and sodium hydroxide buffer (pH 4.2): poloxamer: glycerol: polyethylene glycol 400: sodium alginate=70:3:18:1:1:3 : 0.3 (mass ratio W / W)
[0177] 2. Preparation process
[0178] (1) Weigh 180g of citric acid and sodium hydroxide buffer (pH 4.2), 700g of refined sugar, 30g of neomycin sulfate, 10g of poloxamer, 10g of glycerin, 30g of polyethylene glycol 400, and 3g of sodium alginate ,spare;
[0179] (2) Put the weighed refined white sugar into the tank, add citric acid and sodium hydroxide buffer solution (pH is 4.2), start stirring and heat up until it is completely dissolved, continue to heat up to 120-122°C to keep warm, and keep 15- 20 minutes; then cool down to 80-90°C, keep warm for later use;
[0180] (3) Put the weighed polyethylene glycol 400, glycerin, poloxamer and sodium a...
Embodiment 3
[0183] The preparation of embodiment 3 compound refined white sugar powder
[0184] 1. The proportion of raw and auxiliary materials:
[0185] Refined sugar: kanamycin sulfate: hydroxypropyl cellulose = 70:3:27 (mass ratio W / W)
[0186] 2. Preparation process
[0187] (1) Get 700g of refined white sugar and 270g of hydroxypropyl cellulose of prescription quantity, mix, pulverize, cross 325 mesh medicinal sieves;
[0188] (2) weigh kanamycin sulfate 30g according to the prescription ratio, and set aside;
[0189] (3) The kanamycin sulfate that weighs is put into (1) that has sieved, mixes in mixer;
[0190] (4) Packing, inspection, storage.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com