Method for preparing pelargonic acid and isononanoic acid by catalyzing 1-octylene through water-soluble palladium-phosphine complex catalyst
A catalyst and water-soluble technology, applied in the direction of chemical instruments and methods, carbon monoxide reaction to prepare carboxylic acid, organic compound/hydride/coordination complex catalyst, etc., can solve the problem of polluting products, high catalytic cost, and catalysts that cannot be recycled, etc. problems, to achieve the effect of easy separation, saving production cost and good recycling effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] a. Add 60 mL of deoxygenated water (pH=7), 0.1 g of palladium acetate and 1 g of m-trisulfonate triphenylphosphine ligand to the autoclave in sequence, and stir at room temperature for 1 h after vacuuming;
[0017] b. Add 2 g of phase transfer agent cetyltrimethylammonium bromide, 2 g of p-toluenesulfonic acid and 10 g of substrate 1-octene with a purity of 50%, and seal the autoclave;
[0018] c. Replace the air in the kettle with high-purity CO gas once to reach a reaction temperature of 80°C, and press in CO gas with a pressure of 0.5MPa at one time to carry out a full carbonylation reaction for 2 hours;
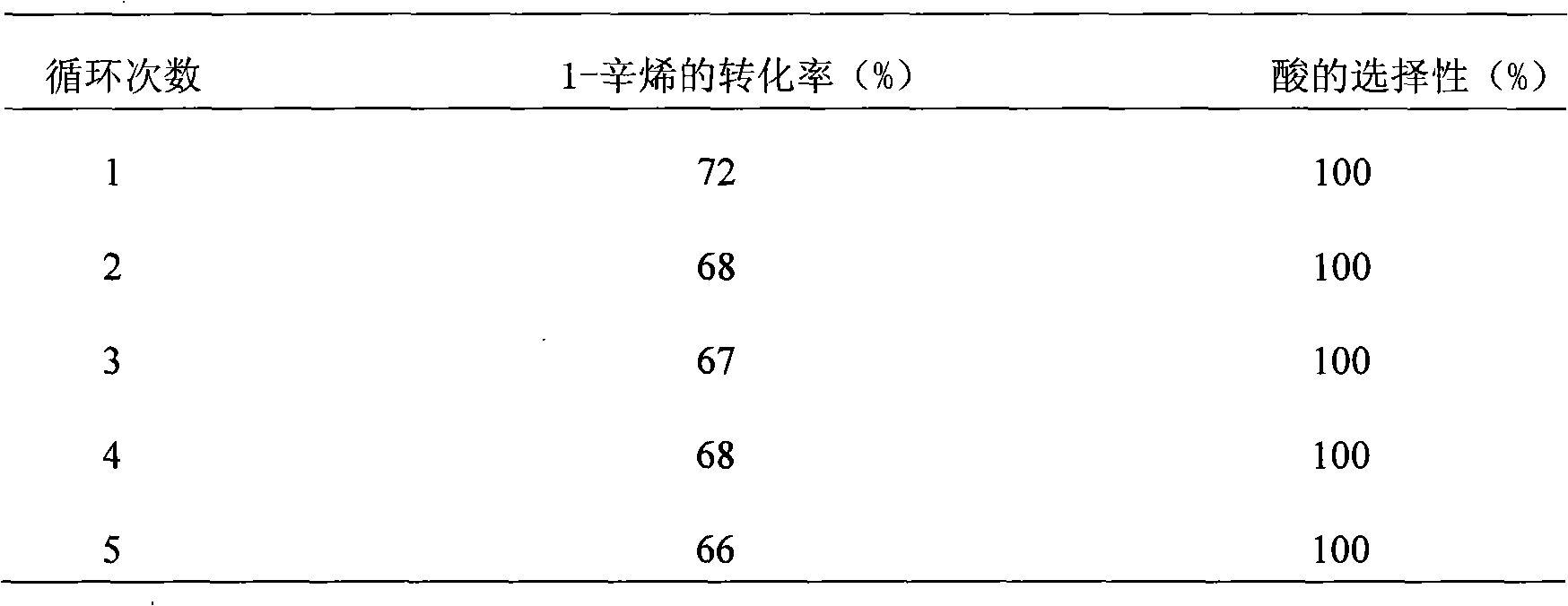
[0019] d. After the reaction is over, fully cool with an ice bath, slowly discharge CO gas, then take out the reactor material, extract the reactor material twice with the organic solvent ethyl acetate, separate the catalyst aqueous phase and the product organic phase, and combine the extracted organic phase solution, the catalyst water phase continues to be recycl...
Embodiment 2
[0021] a. Add 60 mL of deoxygenated water (pH=7), 0.1 g of palladium acetate and 1 g of m-disulfonate triphenylphosphine ligand to the autoclave in turn, and stir at room temperature for 2 h after vacuuming;
[0022] B, add phase transfer agent sodium dodecylbenzene sulfonate 2g again, phosphoric acid 5g and purity are 75% substrate 1-octene 10g, airtight autoclave;
[0023] c. Replace the air in the kettle with high-purity CO gas for 3 times to reach a reaction temperature of 140°C, and press in 6MPa CO gas at one time to carry out a full carbonylation reaction for 12 hours;
[0024] d. After the reaction is completed, fully cool with an ice bath, slowly discharge CO gas, then take out the reactor material, extract the reactor material 4 times with the organic solvent ethyl acetate, separate the catalyst aqueous phase and the product organic phase, and combine the extracted organic phase solution, the catalyst water phase continues to be recycled, and then the combined produc...
Embodiment 3
[0026] a. Add 60 mL of deoxygenated water (pH=7), 0.1 g of palladium chloride and 5 g of m-trisulfonate triphenylphosphine ligand to the autoclave in sequence, and stir at room temperature for 1.5 h after vacuuming;
[0027] b. Add 2 g of cetyltrimethylammonium bromide as a phase transfer agent, 5 mL of concentrated hydrochloric acid and 10 g of substrate 1-octene with a purity of 99%, and seal the autoclave;
[0028] c. Replace the air in the kettle with high-purity CO gas for 5 times. After reaching the reaction temperature of 180°C, press in CO gas at a pressure of 12MPa at one time to carry out a full carbonylation reaction for 20 hours;
[0029] d. After the reaction is completed, fully cool with an ice bath, slowly discharge CO gas, then take out the reactor material, extract the reactor material 6 times with the organic solvent ethyl acetate, separate the catalyst aqueous phase and the product organic phase, and combine the extracted organic phase solution, the catalyst...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap