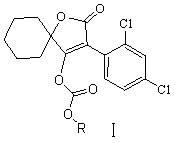
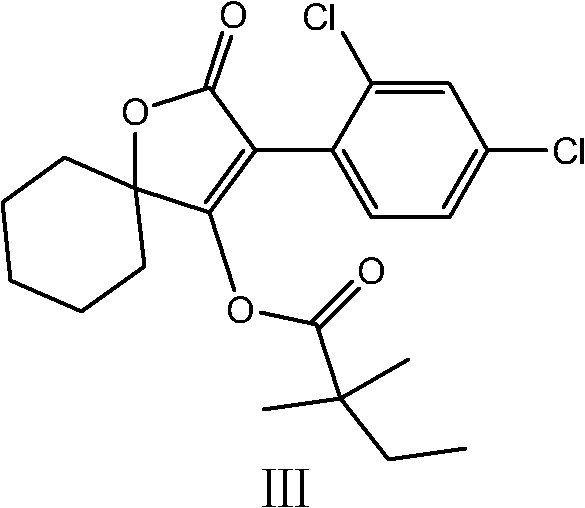
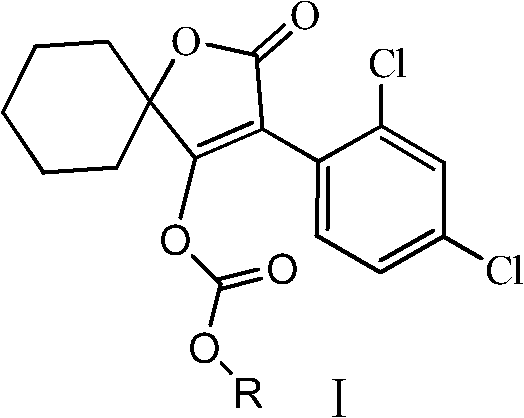
Novel spirodiclofen compound and preparation method and application thereof
A technology of ester compounds and compounds, applied in the field of pesticides, can solve the problems of the preparation method of new spirodiclofen compounds and the acaricidal activity that have not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1. A method for synthesizing a novel spirodiclofen compound, the reaction is:
[0026]
[0027] Add 60ml dichloromethane and 6.3g (0.02mol) 3-(2,4-dichlorophenyl)-2-oxygen to a 100ml reaction flask equipped with electromagnetic stirring, condenser, constant pressure dropping funnel and thermometer. Substitute-1-oxaspiro[4,5]-dec-3-en-4-ol and 7.5g (0.075mol) triethylamine, stir to dissolve, add sec-butyl chloroformate 3.3g (0.024 mol), then continue to stir at room temperature, and the thin layer plate controls the reaction time. After the reaction is over, pour the reaction solution into saturated NaHCO 3 Solution (pH about 8), stir for ten minutes, separate, wash the organic layer with 40-60ml of water, and use anhydrous Na for the organic phase 2 SO 4 After drying, the solvent dichloromethane was removed under reduced pressure to obtain a light yellow viscous liquid, which was recrystallized with 50 ml of methanol to obtain 7.08 g of colorless crystals (namely I...
Embodiment 2
[0029] Example 2. A method for synthesizing a new type of spirodiclofen, the reaction is:
[0030]
[0031] Add 60ml 1,2-dichloroethane and 6.3g (0.02mol) 3-(2,4-dichlorophenyl) into a 100ml reaction flask equipped with electromagnetic stirring, condenser, constant pressure dropping funnel and thermometer. )-2-oxo-1-oxaspiro[4,5]-dec-3-en-4-ol and 7.5g (0.075mol) triethylamine, stir to dissolve, add n-butyl chloroformate dropwise under ice water bath Ester 3.3g (0.024mol), then continue to stir at room temperature, and the thin layer plate controls the reaction time. After the reaction is over, pour the reaction solution into saturated NaHCO 3 Solution (pH about 8), stir for ten minutes, separate, wash the organic layer with 40-60ml of water, and use anhydrous Na for the organic phase 2 SO 4 After drying, the solvent 1,2-dichloromethane was removed under reduced pressure to obtain a pale yellow oily liquid, which was recrystallized with 50 ml of methanol to obtain 6.59 g of color...
Embodiment 3
[0033] Example 3. A method for synthesizing a novel spirodiclofen compound, the reaction is:
[0034]
[0035] In a 100ml reaction flask equipped with electromagnetic stirring, condenser, constant pressure dropping funnel and thermometer, 60ml benzene, 6.3g (0.02mol) 3-(2,4-dichlorophenyl)-2-oxo- 1-oxaspiro[4,5]-dec-3-en-4-ol and 5.9g (0.075mol) pyridine, after stirring to dissolve, 3.6g (0.024mol) isoamyl chloroformate was added dropwise under ice water bath, then Continue stirring at room temperature, and the thin layer plate controls the reaction time. After the reaction is over, pour the reaction solution into saturated NaHCO 3 Solution (pH about 8), stir for ten minutes, separate, wash the organic layer with 40-60ml of water, and use anhydrous Na for the organic phase 2 SO 4 After drying, the solvent benzene was removed under reduced pressure to obtain a pale yellow oily liquid, which was recrystallized with 50 ml of methanol to obtain 6.9 g of colorless crystals (namely I c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com