Preparation method of exterior sodium dick acid anhydride
A nadic acid anhydride and exterior technology, applied in the field of organic chemical synthesis, can solve problems such as environmental pollution, reduce production costs, reduce solvent pollution, and strengthen deep processing and utilization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
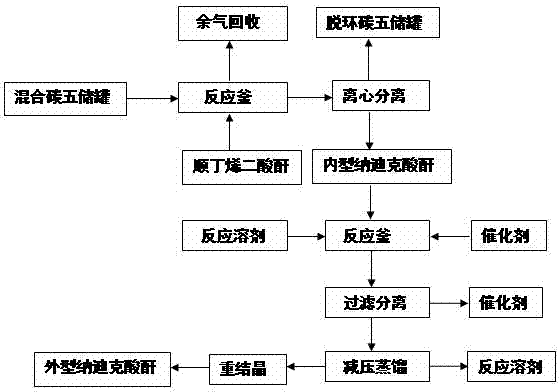
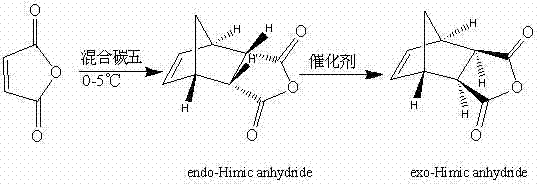
[0018] Under stirring conditions at 0°C, gradually add maleic anhydride to 60 g of mixed carbon five, stop the reaction when there is no cyclopentadiene detected by GC, add 20.9 g of maleic anhydride, and filter with suction to obtain 33.2 g internal Nadic acid anhydride, melting point 160-162 ℃, purity 98% (GC), yield 95%. 1 H NMR (300Hz, CDCl 3 ),δ / ppm: 6.313(2H, t, J=1.778Hz, 5-H, 6-H), 3.571-3.611(2H, m, 2-H, 3-H), 3.496-3.528(2H, m , 1-H, 4-H), 1.765-1.806(1H, m, 7-H), 1.572-1.604(1H, dd, J 1 =0.51Hz,J 2 =9.0Hz, 7-H); 13 C NMR (300Hz, CDCl 3 ), δ / ppm: 171.4, 135.4, 52.7, 47.0, 46.0.
[0019] Add the above-mentioned 33.2 g of endo-nadic anhydride, 200 mL of ethanol as solvent, 6.6 g of urotropine, 16.6 mg of benzoyl peroxide and 2.3 g of [Cu(dppm)(NO 3 )] 2 As a catalyst, after 10 h of reflux reaction, the catalyst was removed by filtration, and the solvent was recovered under reduced pressure. The mixed solvent of 100 mL toluene and 100 mL acetone was crystallized ...
Embodiment 2
[0021] Under stirring conditions at 2°C, gradually add maleic anhydride to 300 g of mixed carbon five, stop the reaction when no cyclopentadiene is detected by GC, add 105 g of maleic anhydride in total, and centrifuge the reaction mixture, 168 g of endo-nadic anhydride was obtained, with a melting point of 162-164°C, a purity of 99% (GC), and a yield of 95%.
[0022] Add the above-mentioned 168 g of internal Nadic anhydride, 800 mL of isopropanol as solvent, 16.8 g of urotropine, 50.4 mg of benzoyl peroxide and 16.8 g of [Cu(dppm)(NO 3 )] 2 As a catalyst, after 24 h of reflux reaction, the catalyst was removed by filtration, and the solvent was recovered under reduced pressure. The mixed solvent of 400 mL toluene and 400 mL acetone was crystallized to obtain 163 g of pure exo-nadic acid anhydride, with a melting point of 140-142 °C and a purity of 98% (GC) , yield 97%.
Embodiment 3
[0024] Under stirring conditions at 5°C, gradually add maleic anhydride to 1800 g of mixed carbon five, stop the reaction when there is no cyclopentadiene detected by GC, add 630 g of maleic anhydride in total, and centrifuge the reaction mixture, 1013 g of endo-nadic anhydride was obtained, with a melting point of 160-162 °C, a purity of 98% (GC), and a yield of 96%.
[0025] In the reactor, add the above-mentioned 1013 g internal Nadic anhydride, 5 L propanol as a solvent, 304 g of urotropine, 0.81 g of benzoyl peroxide and 40.6 g of [Cu (dppm) (NO 3 )] 2 As a catalyst, after 16 hours of reflux reaction, the catalyst was removed by filtration, the solvent was recovered under reduced pressure, and the mixed solvent of 2 L toluene and 2 L acetone was recrystallized to obtain 973 g of pure Nadic anhydride, with a melting point of 140-142 °C and a purity of 98% (GC ), yield 96%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com