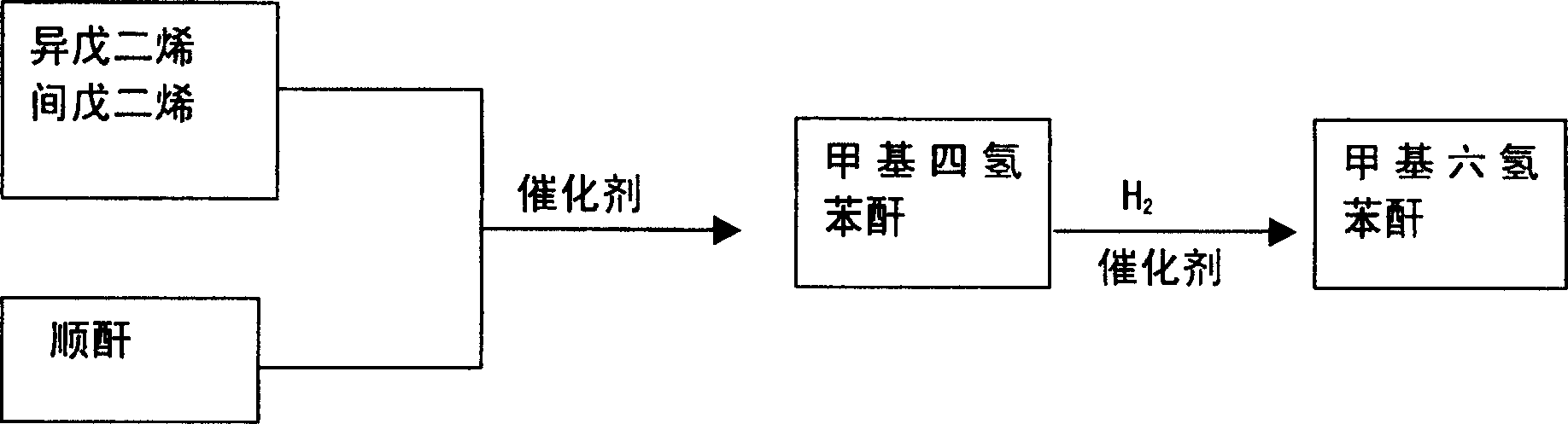
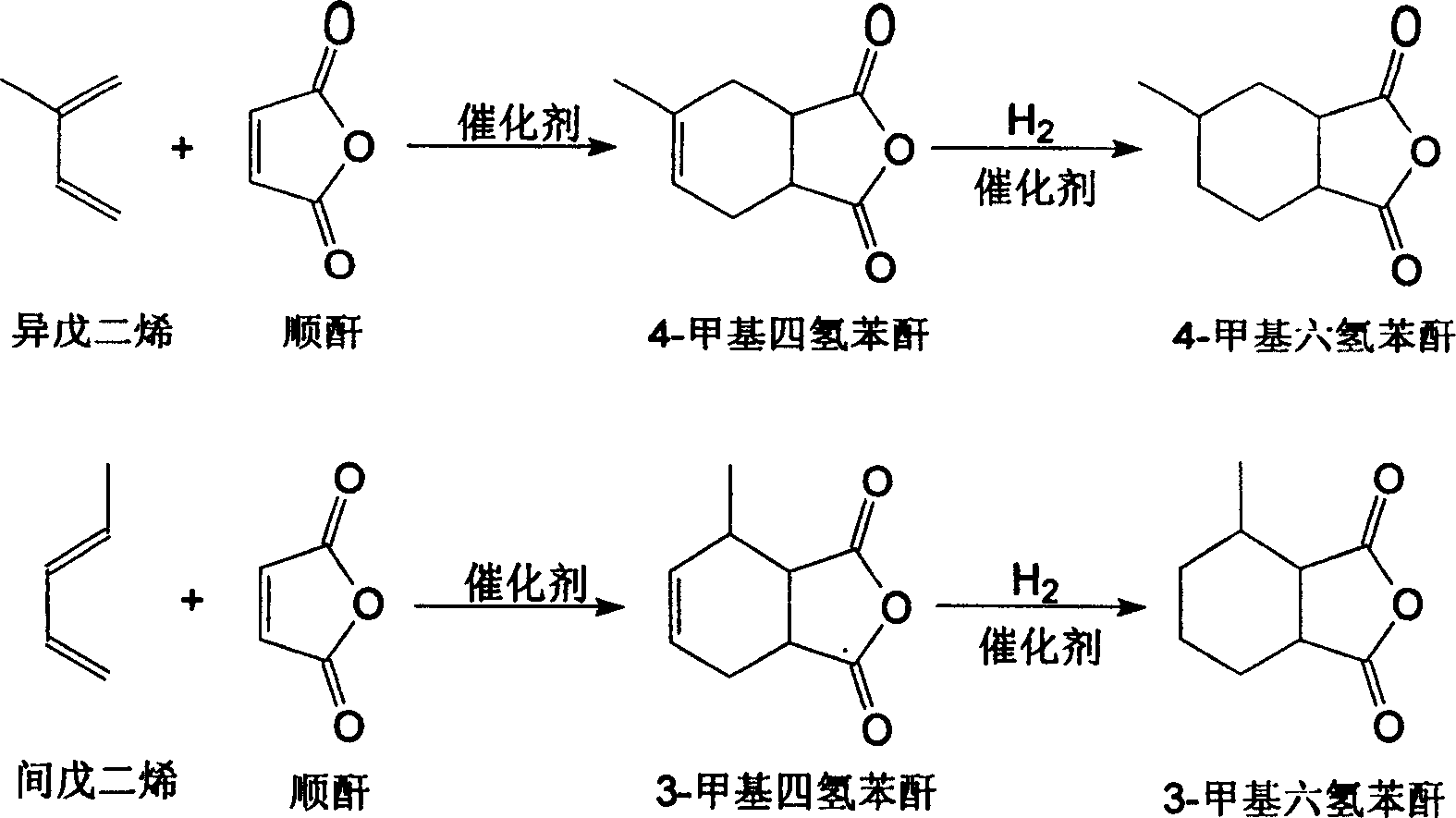
Novel method for producing methyl hexahydrobenzene anhydride
A technology of methyl hexahydrophthalic anhydride and methyl tetrahydrophthalic anhydride, applied in the new field of producing methyl hexahydrophthalic anhydride, can solve problems such as easy coking, high boiling point, catalyst poisoning, etc. The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] At a temperature of 50°C and the presence of 3 grams of aluminum chloride, the acyclic carbon five (the main components of which are isoprene and piperylene) is gradually added to 98 grams of maleic anhydride, and the Diels-Alder reaction is carried out. Generates methyl tetrahydrophthalic anhydride. Then add 1 g of skeleton nickel and 0.1 g of bis(bis(diphenylphosphinooxyethane)) copper tetrabromide polymer to the generated methyltetrahydrophthalic anhydride, and place it in an autoclave at a temperature of 140°C and a hydrogen pressure of 4MPa. The catalytic hydrogenation reaction was carried out for 4 hours, the catalyst was filtered after cooling, and 152 grams of colorless liquid methylhexahydrophthalic anhydride was obtained by distillation under reduced pressure, with a yield of 90.3%.
Embodiment 2
[0021] In the presence of a temperature of 80° C. and 1.5 g of aluminum chloride, acyclic carbon five (the main components of which are isoprene and piperylene) is gradually added to 98 g of maleic anhydride to react to generate methyltetrahydrophthalic anhydride. Then, 2.5 grams of skeleton nickel and 0.3 grams of bis(bis(diphenylphosphinooxyethane)) copper tetrabromide polymer were added to the generated methyltetrahydrophthalic anhydride, and placed in an autoclave at a temperature of 120°C and a hydrogen pressure of 2MPa. The catalytic hydrogenation reaction was carried out for 5 hours, the catalyst was filtered after cooling, and 157 grams of colorless liquid methylhexahydrophthalic anhydride was obtained by distillation under reduced pressure, with a yield of 93.3%.
Embodiment 3
[0023] At a temperature of 120° C. and the presence of 0.5 g of zinc chloride, acyclic carbon five (the main components of which are isoprene and piperylene) is gradually added to 98 g of maleic anhydride to react to generate methyltetrahydrophthalic anhydride. Then add 5 g of skeleton nickel and 0.5 g of bis(bis(diphenylphosphinooxyethane)) copper tetrabromide polymer to the generated methyltetrahydrophthalic anhydride, and place it in an autoclave at a temperature of 100°C and a hydrogen pressure of 1MPa. The catalytic hydrogenation reaction was carried out for 6 hours, the catalyst was filtered after cooling, and 155 g of colorless liquid methylhexahydrophthalic anhydride was obtained by distillation under reduced pressure, with a yield of 92.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com