Endo-methylene hexahydrophthalic anhydride and production method thereof
A technology of endomethylene hexahydrophthalic anhydride and production method, which is applied in the field of organic chemical synthesis, can solve the problems of insufficient development and utilization, and achieve the convenience of industrial production, reduce production costs, and reduce environmental pollution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
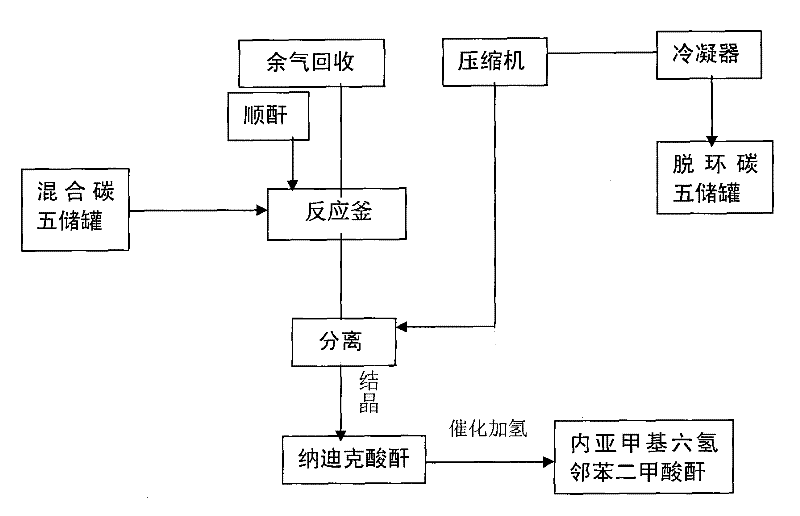
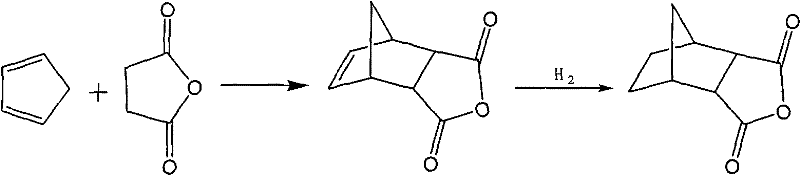
[0014] Under 0℃ and stirring conditions, gradually add maleic anhydride to 600g of mixed carbon five, stop the reaction when there is no cyclopentadiene detected by gas chromatography, add 209g of maleic anhydride, filter with suction, and crystallize the solid Obtain 290 g of Nadic anhydride with a yield of 83%.
[0015] Then, 8.7g of catalyst was added to the generated Nadic anhydride (the loading amount of Ni was 10wt.%, n Co : N Ni =1:100), the catalytic hydrogenation reaction was carried out in an autoclave at a temperature of 140°C and a hydrogen pressure of 3MPa for 4 hours. After cooling, the catalyst was filtered and distilled under reduced pressure to obtain 261 g of endomethylene hexahydrophthalic anhydride. The yield was 90%.
[0016] 1 HNMR(300MHz, CDCl 3 ), δ / ppm: 3.15 (2H, CH), 2.13 (2H, CH), 1.77 (1H, CH), 1.74 (2H, CH) 2 ), 1.52(1H, CH), 1.47(2H, CH) 2 );
[0017] 13 CNMR(100MHz, CDCl 3 ), δ / ppm: 171.4, 47.2, 39.2, 34.5, 27.2;
[0018] IR(KBr), v / cm -1 : 2948, 1894...
Embodiment 2
[0021] Under stirring conditions at 2°C, gradually add maleic anhydride to 6Kg of mixed carbon five, stop the reaction when there is no cyclopentadiene detected by gas chromatography, add 2.1Kg of maleic anhydride, and centrifuge the reaction mixture. The liquid is vaporized, compressed and condensed to obtain 4.8Kg of decyclic carbon five, and the solid is crystallized to obtain 2.6Kg of Nadic anhydride, with a yield of 73%.
[0022] Then, 78g of catalyst (Ni loading is 20wt.%, n Co : N Ni =1:50), the catalytic hydrogenation reaction was carried out in an autoclave at a temperature of 110°C and a hydrogen pressure of 4MPa for 5 hours. After cooling, the catalyst was filtered and distilled under reduced pressure to obtain 2.4Kg of endomethylene hexahydrophthalic anhydride. The rate is 92%.
Embodiment 3
[0024] Under stirring conditions at 5°C, gradually add maleic anhydride to 18Kg of mixed carbon five, stop the reaction when there is no cyclopentadiene detected by gas chromatography, add 6.3Kg of maleic anhydride, and centrifuge the reaction mixture. The liquid is vaporized, compressed and condensed to obtain 13.8Kg of acyclic carbon five, and the solid is crystallized to obtain 8.4Kg of Nadic anhydride, with a yield of 79%.
[0025] Then, 252g of catalyst was added to the generated Nadic anhydride (the loading amount of Ni was 40wt.%, n Co : N Ni =1:200), the catalytic hydrogenation reaction was carried out in an autoclave at a temperature of 130°C and a hydrogen pressure of 1MPa for 6 hours. After cooling, the catalyst was filtered and distilled under reduced pressure to obtain 7.5Kg of endomethylene hexahydrophthalic anhydride. The rate is 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com