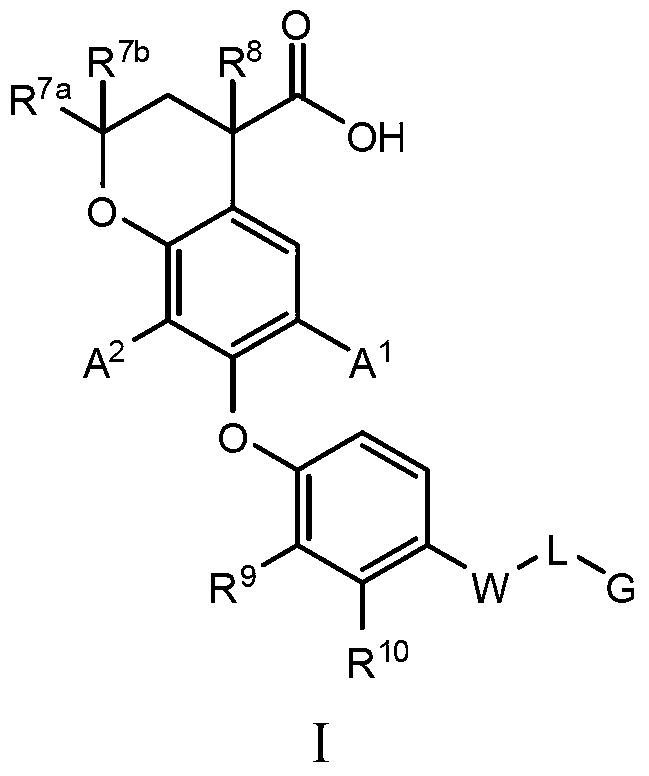
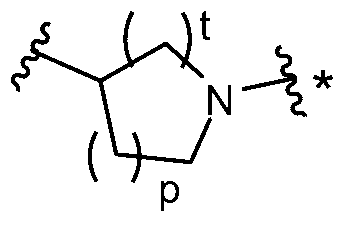
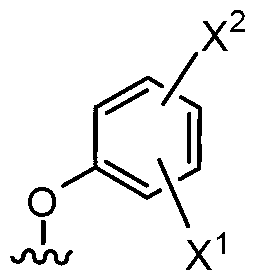
6-substituted phenoxychroman carboxylic acid derivatives
A substituent, phenyl technology, applied in the field of novel compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0590] DP-2 binding inhibition assay
[0591] The coding sequence for human DP2 was introduced into the human leukemia cell line K562 by electroporation and stable clones expressing DP2 were obtained by limiting dilution followed by staining of the cell surface with a rat monoclonal antibody specific for human DP2. Membranes were prepared from one of these DP2 expressing clones and used to determine the ability of compounds of the invention to inhibit the binding of prostaglandin D2 (PGD2) to its receptor DP2 by the following procedure in the presence of one or more of the following serum protein concentrations: The concentration is 0.1% BSA, 1% HSA or 4% HSA. Cell membranes (1.25 μg / well for 0.1% BSA and 6 μg / well for 1% or 4% HSA) were mixed with vitrified 96-well U-bottom polypropylene plates. 3 H-labeled PGD 2 and various concentrations of test compounds in 150 microliters of binding buffer (50mM Tris-HCl (pH7.4), 40mM MgCl 2 , 0.1% fetal bovine serum albumin, 0.1% NaN...
Embodiment B
[0602] Mouse model of allergic rhinitis
[0603] Allergic rhinitis (AR) is the most common form of atopy, with an estimated prevalence of 5% to 22% (Naclerio, R.M., N. Engl. J. Med. 1991, 325:860-869) , which results in huge associated costs for treatment. Typical symptoms of AR in human patients are well known, mainly sneezing and nasal congestion (Corrado O.J., et al., Br.J.Clin.Pharmacol.1987, 24:283-292; Mygind N and Anggard A.Clin Rev. Allergy, 1984, 2:173-188). The three main causes of nasal congestion are considered to be the direct result of dilation of volume vessels in the nasal septum and turbinates, edematous swelling of the nasal membranes, and secretions (Sherwood J.E., et al.J. Allergy Clin. Immunol., 1993, 92:435- 441; Juliusson S. and Bende M., Clin Allergy 1987, 17: 301-305; Mygind N. et al., Eur J Respir Dis Suppl. 1987, 153: 26-33; Gawin A. Z., et al., J Appl Physiol .1991, 71:2460-2468).
[0604] Nasal reactivity in AR has been demonstrated to occur ...
preparation example 1
[0614] 4 - (6 - chlorine - 4 - (Ethoxycarbonyl)chroman - 7 - oxy)benzoic acid
[0615]
[0616] Step A: Preparation of 3-chloro-1-(5-chloro-2,4-dihydroxyphenyl)propan-1-one: Trifluoromethanesulfonic acid (500 g, 3.33 mol) was charged to 2 L of 4- Neck round bottom flask and cool the contents of the flask to below 10°C. 4-Chlororesorcinol (100 g, 0.69 mol) was added in portions over 20-30 minutes, maintaining the temperature at 4-8 °C. The reaction mixture was stirred at or below 10°C until a clear solution formed (40 minutes). 3-Chloropropionic acid (78.8 g, 0.73 mol) was heated until melted and then added dropwise in liquid form to the bottle over 45 minutes maintaining the temperature at or below 10°C. The reaction mixture was stirred at or below 10°C for an additional 10 minutes, then slowly heated to 50-55°C and maintained at this temperature for 6 hours. The reaction mixture was cooled to ambient temperature and added dropwise to water (1.1 L) contained in a 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com