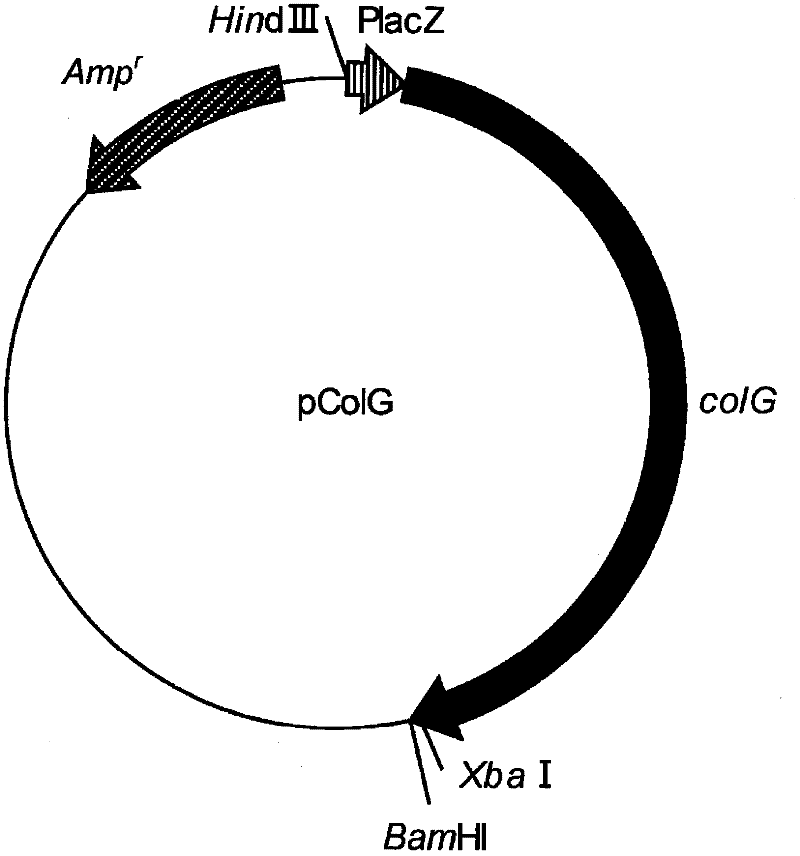
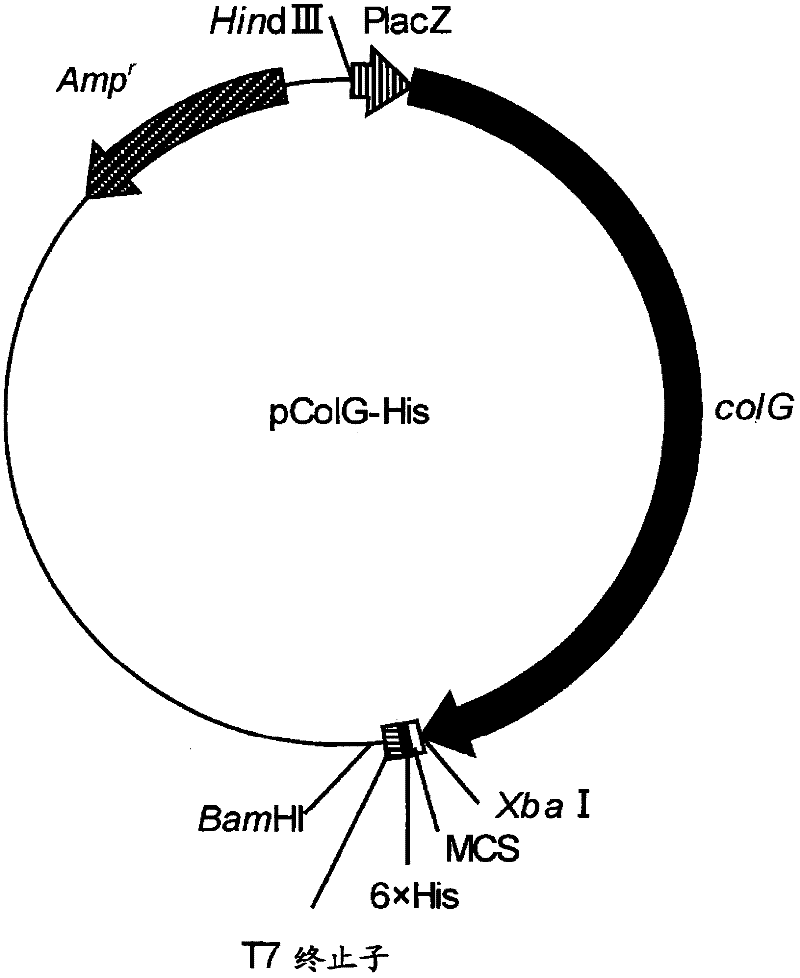
Fusion collagenase to which affinity tag is attached, and method for producing same
A technology of collagenase and marker, applied in the field of collagenase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] [ Example 1 ] Expression of a fusion collagenase with a histidine tag attached to the carboxyl terminus of collagenase G derived from Clostridium histolytica (fusion collagenase G)
[0084] (1-1) Preparation of collagenase G gene fragment
[0085] The 5' side end to the 3' side end of the Clostridium histolyticum-derived collagenase G gene were amplified by PCR using the Clostridium histolyticum-derived genomic DNA as a template. At this time, it is designed to form at the 3' side end of the amplified collagenase G gene Xba I recognize the sequence, and add the stop codon linked to the gene Bam Primers for the HI recognition sequence. As a result, mutations were introduced at two positions (amino acids at positions 1007 and 1008 in SEQ ID NO: 1) of the carboxy-terminal amino acid sequence of native collagenase G described in Non-Patent Document 1, and the sequence numbers were changed to: The amino acid sequence described in 2.
[0086] Primers used in this PCR ...
Embodiment 2
[0112] [ Example 2 ]Cultivation of Fusion Collagenase G Expressing Escherichia coli and Selective Recovery of Collagenase G with CBD
[0113] (2-1) Culture of Escherichia coli expressing fusion collagenase G
[0114] The fused collagenase G-expressing Escherichia coli obtained in Example 1 was inoculated into a 250 ml Erlenmeyer flask supplemented with 100 ml of medium, and cultured with stirring at 200 rpm at 28° C. for 16 hours. The culture medium used in this cultivation is TB culture medium (1.2% tryptone, 2.4 % yeast extract, 0.94% dipotassium hydrogen phosphate, 0.22% potassium dihydrogen phosphate, 0.8% glycerin).
[0115] (2-2) Preparation of Extracts of Collagenase G Expressing Escherichia coli
[0116]The culture-terminated solution obtained in (2-1) was centrifuged to recover bacterial cells, and the recovered bacterial cells were lysed with 10 ml of POPculture Regent (manufactured by Merck Corporation) to extract proteins in the bacterial cells. In order to re...
Embodiment 3
[0121] [ Example 3 ] Expression of a fusion collagenase with a histidine tag linked to the carboxyl terminus of collagenase H derived from Clostridium histolyticum
[0122] (3-1) Preparation of Collagenase H Gene Fragment
[0123] The 5' side end to the 3' side end of the Clostridium histolyticum-derived collagenase H gene were amplified by PCR using the Clostridium histolyticum-derived genomic DNA as a template. At this time, it is designed to form at the 3' side end of the amplified collagenase H gene Xba I recognize the sequence, and add the stop codon linked to the gene Bam Primers for the HI recognition sequence. As a result, a mutation was introduced at one position (amino acid at position 980 of SEQ ID NO: 5) in the carboxy-terminal amino acid sequence of the amino acid sequence of native collagenase H described in Non-Patent Document 2, and changed to SEQ ID NO: 6 The amino acid sequence described in.
[0124] Primers used in this PCR are as follows.
[0125] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com