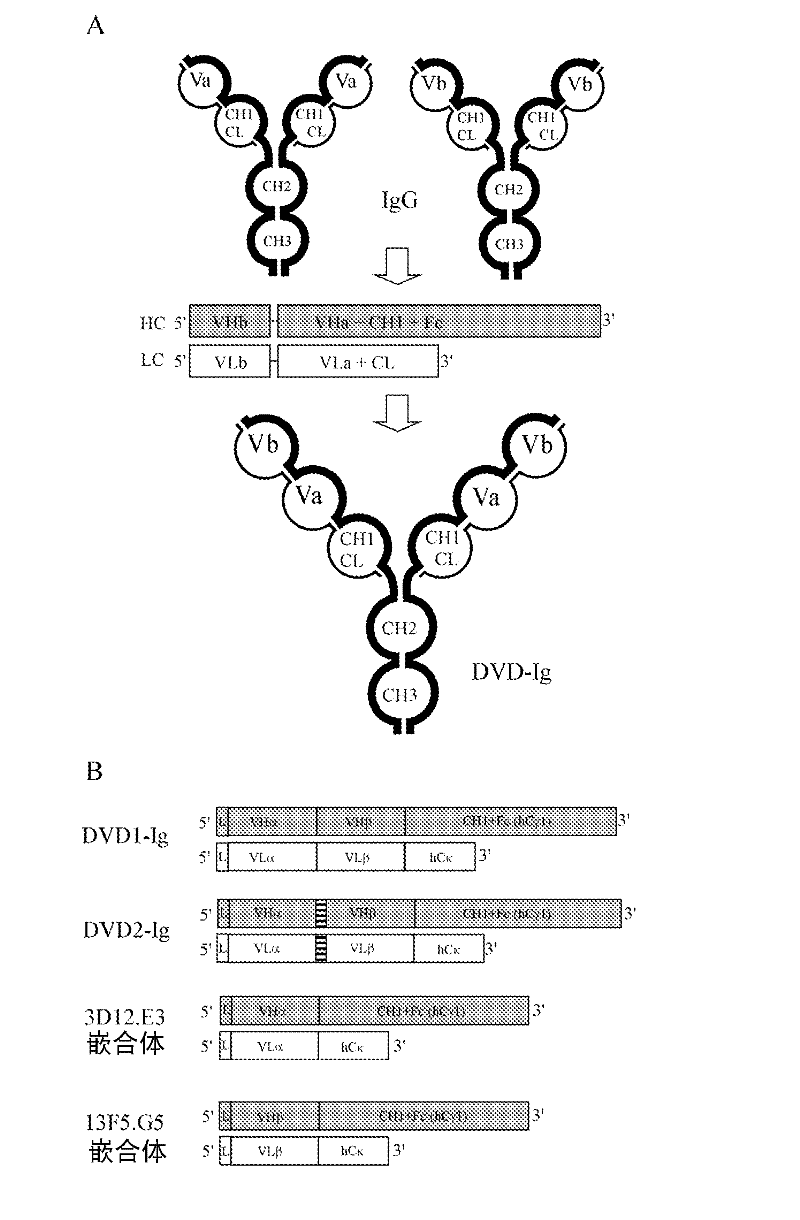
Dual variable domain immunoglobulins and uses thereof
A domain-binding protein technology for the prevention and/or treatment of acute and chronic inflammatory diseases, diagnosis, cancer and other diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
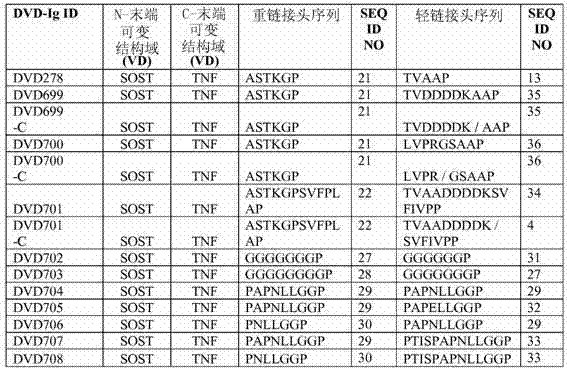
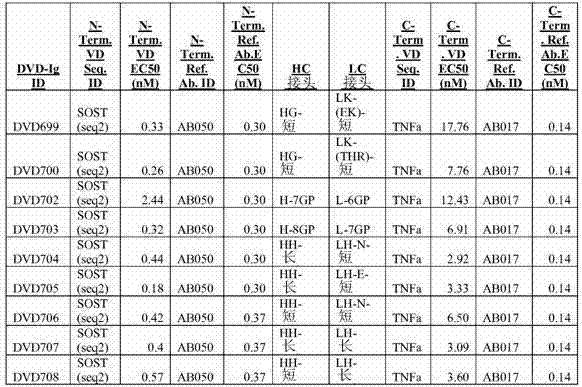
[0498] Example 1: Design, construction and analysis of DVD-Ig
Embodiment 11
[0499] Example 1.1: Proteolytic cleavage of DVD-Ig with cleavable linkers
[0500] Enterokinase-cleaved DVD699 (denoted DVD699-C) and DVD701 (denoted DVD701-C) protein samples were generated as follows. Mix 180 μL of purified DVD-Ig at 1.1 mg / ml with 20 μL of 10X enterokinase cleavage buffer (500 mM Tris-HCl (pH 8.0), 10 mM CaCl 2 , 1% Tween-20) combination. 5 U / mg of EKMax (Invitrogen, Cat. No. E180-01) was added to the mixture, and the mixture was incubated at room temperature for 2 days. To confirm that all light chains were processed at the engineered site, samples (reduced and non-reduced) were run on SDS-PAGE before and after cleavage, and when the ~36 kDa band was no longer present in the reduced sample The reaction was considered complete when the ~24 kDa and ~12 kDa bands (corresponding to the expected cleaved LC fragments) were present instead. To confirm that the 12 kDa fragment remained bound to the cleaved DVD-Ig molecule, the mixture was additionally purified ...
Embodiment 12
[0508] Example 1.2: Assays for identification and characterization of parental antibodies and DVD-Ig
[0509] Unless otherwise indicated, the following assays were used throughout the Examples to identify and characterize the parental antibodies and DVD-Igs.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com