Curable resin composition and cured products thereof
A technology of curable resin and composition, applied in the field of liquid curable resin composition, to achieve the effects of excellent low coloring, excellent refractive index, and excellent storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1~1-4
[0109][Examples 1-1 to 1-4 and Comparative Examples 1-1 to 1-3] Production of curable resin compositions No.1 to No.4 and comparative curable resin compositions No.1 to No.3
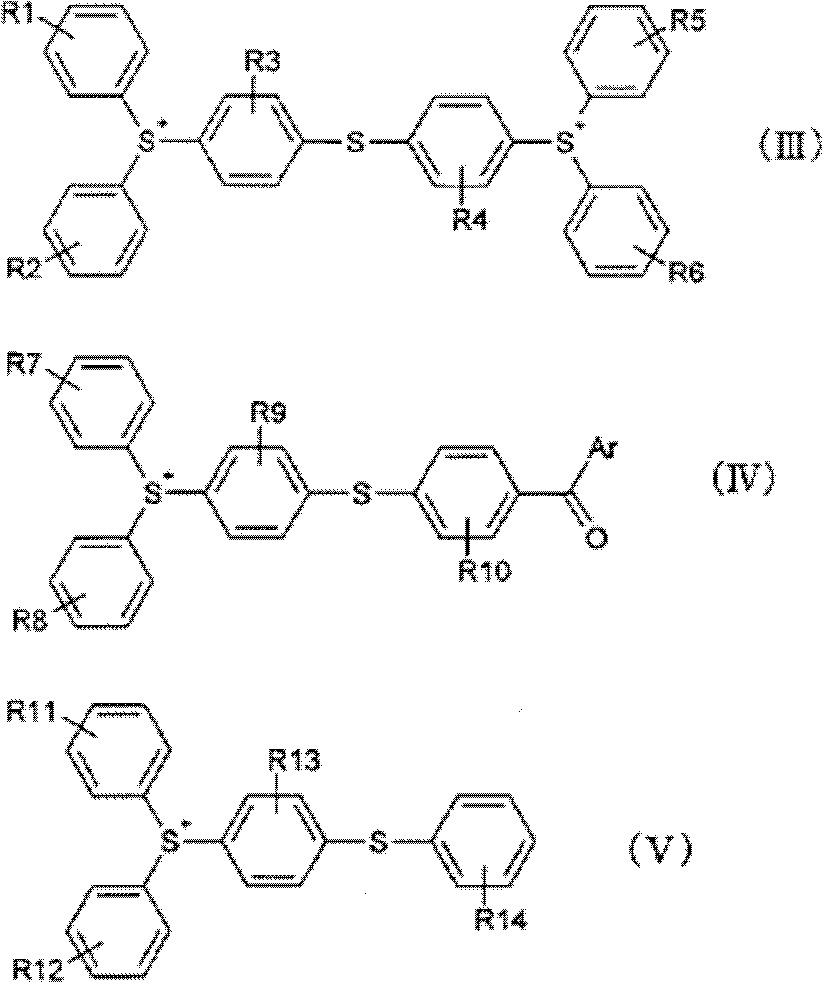
[0110] According to Table 1, heat the following (1-1) to (1-3) epoxy compounds and (2) diluent to 80°C to dissolve them, then cool down to 60°C, add (3-1) and (3-2 ) The energy ray-sensitive cationic polymerization initiator was completely dissolved to prepare curable resin compositions No. 1 to No. 4 and comparative curable resin compositions No. 1 to No. 3, respectively.
[0111] [complex]
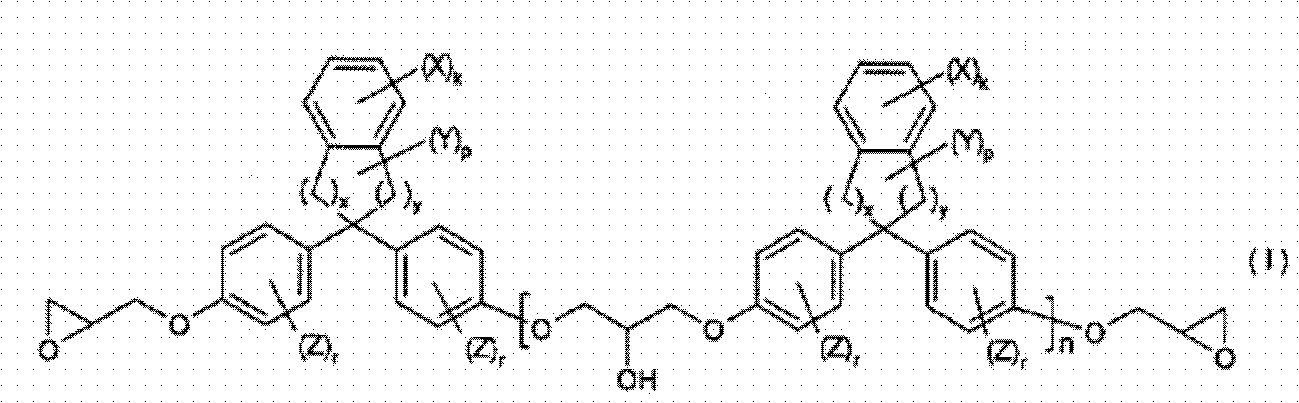
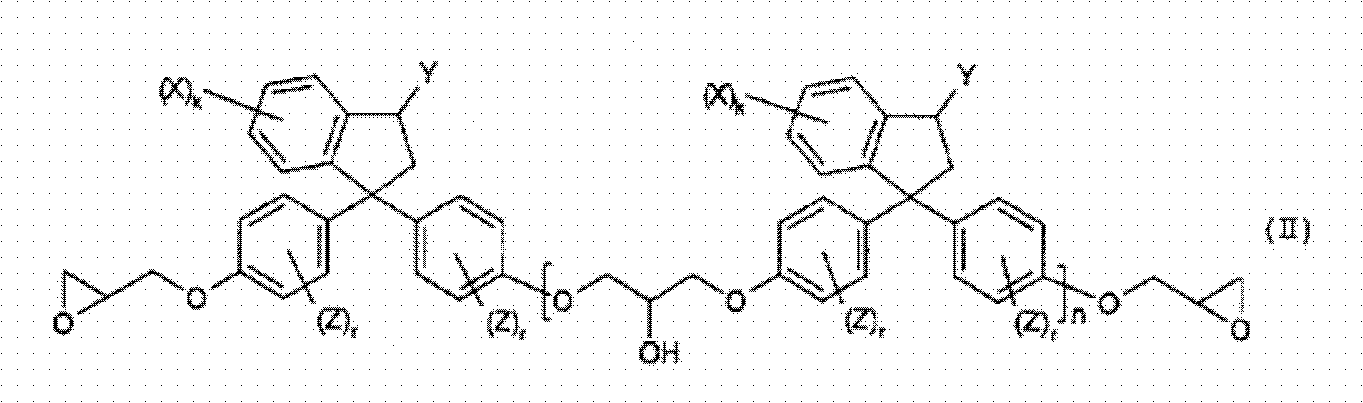
[0112] (1-1) 1,1-bis(4-(2,3-epoxypropoxy)phenyl)-3-phenylindane (corresponding to the compound when n=0 of the above-mentioned compound No.40 ), hereinafter referred to as epoxy compound No.1.
[0113] Epoxy compound No.1
[0114] (1-2) 1,1-bis(4-(2,3-epoxypropoxy)phenyl)-3,5-diphenylindane (equivalent to n=0 of the above compound No.31 When the compound), hereinafter referred to as epoxy compound No.2.
[0...
Embodiment 2-1~2-4 and comparative example 2-1 and 2-2
[0133] [Examples 2-1 to 2-4 and Comparative Examples 2-1 and 2-2] Production of Cured Products No.1 to No.4 and Comparative Cured Products No.2 and No.3
[0134] The curable resin compositions and comparative curable resin compositions obtained in Examples 1-1 to 1-4 and Comparative Examples 1-2 and 1-3 were heated to 60°C, respectively, and coated on the mold release treatment. on a glass substrate. Together with a 1.00mm spacer, use a piece of glass to clamp and paste, and use a high-pressure mercury lamp to 3000mJ / cm 2 (total 6000mJ / cm 2 ) After exposing one side of the glass, it was treated at 150° C. for 2 hours to be cured. After cooling to room temperature, the cured product was peeled off from the glass to prepare cured product No.1 to No.4 and comparative cured product No.2 and No.3.
[0135] [Examples 3-1 to 3-4 and Comparative Examples 3-1 and 3-2] Evaluation of Cured Products No.1 to No.4 and Comparative Cured Products No.2 and No.3
[0136] The "refractive ind...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com