Multispecific antibodies comprising full length antibodies and single chain fab fragments
A multi-specific antibody and full-length antibody technology, applied in the direction of antibodies, antibody mimics/scaffolds, anti-receptors/cell surface antigens/cell surface determinant immunoglobulins, etc., can solve problems such as low expression yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0248] Design of Multispecific Antibody Molecules According to the Present Invention Recognizing Human IGF1 Receptor and Human EGF Receptor
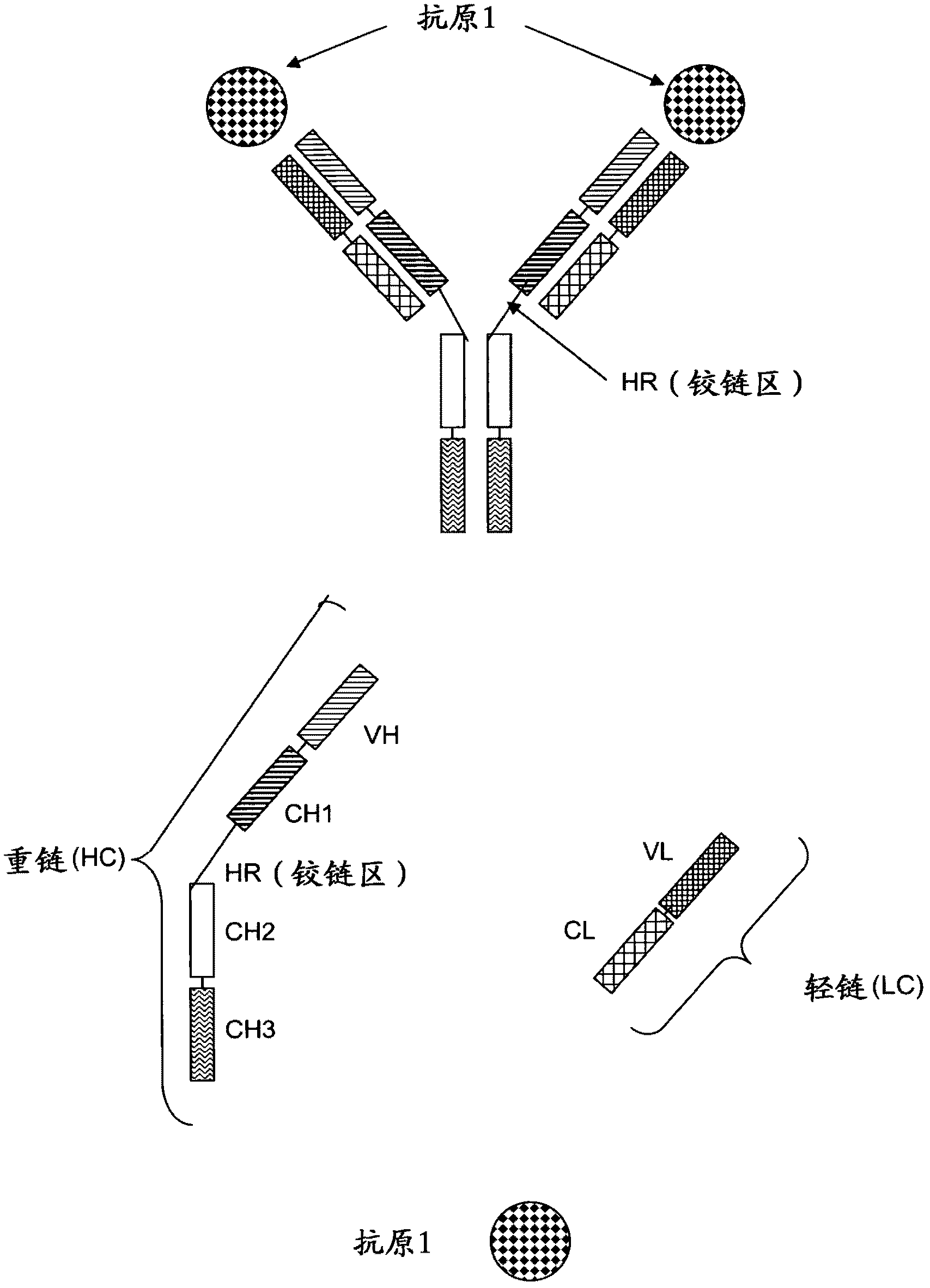
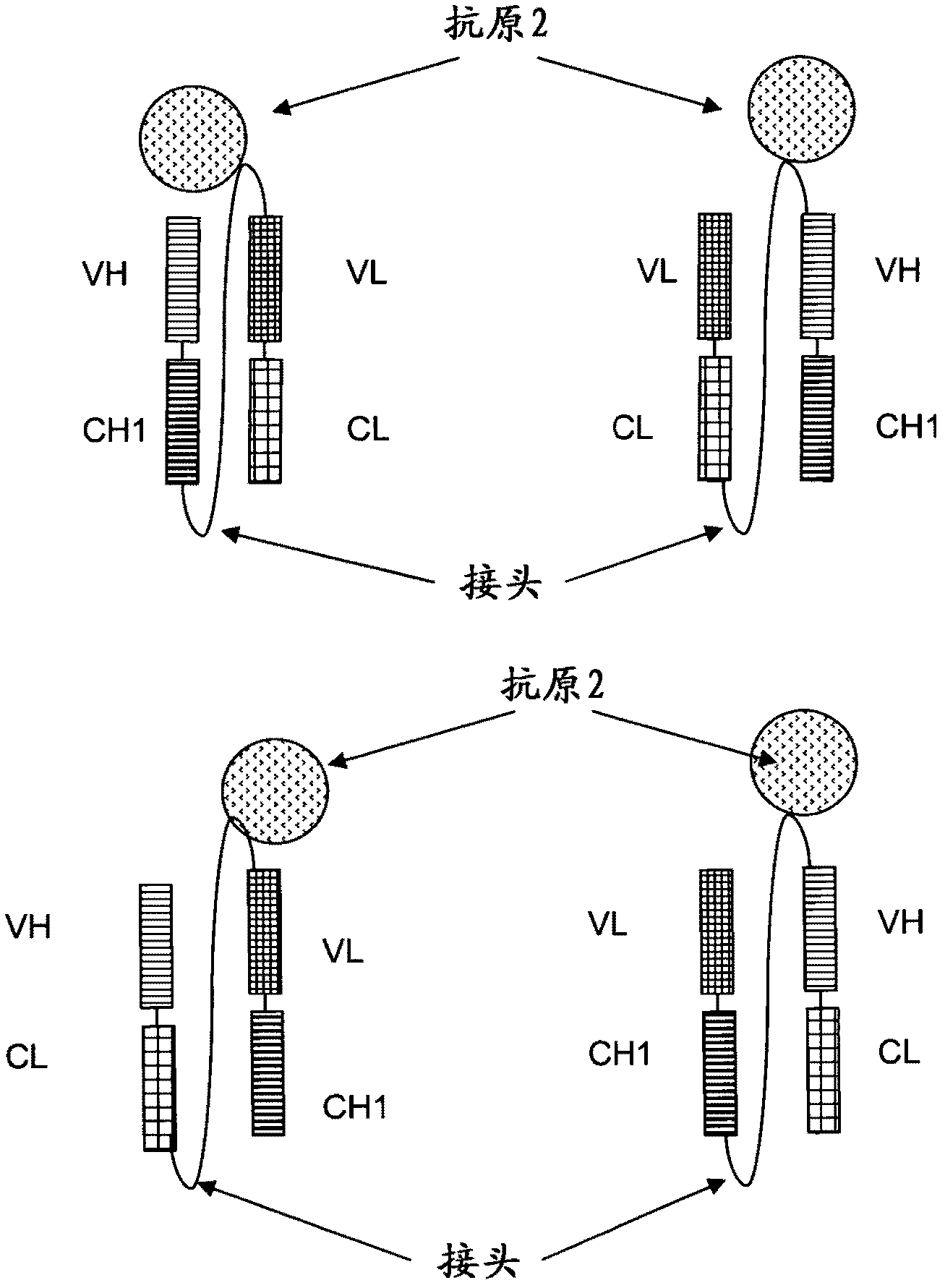
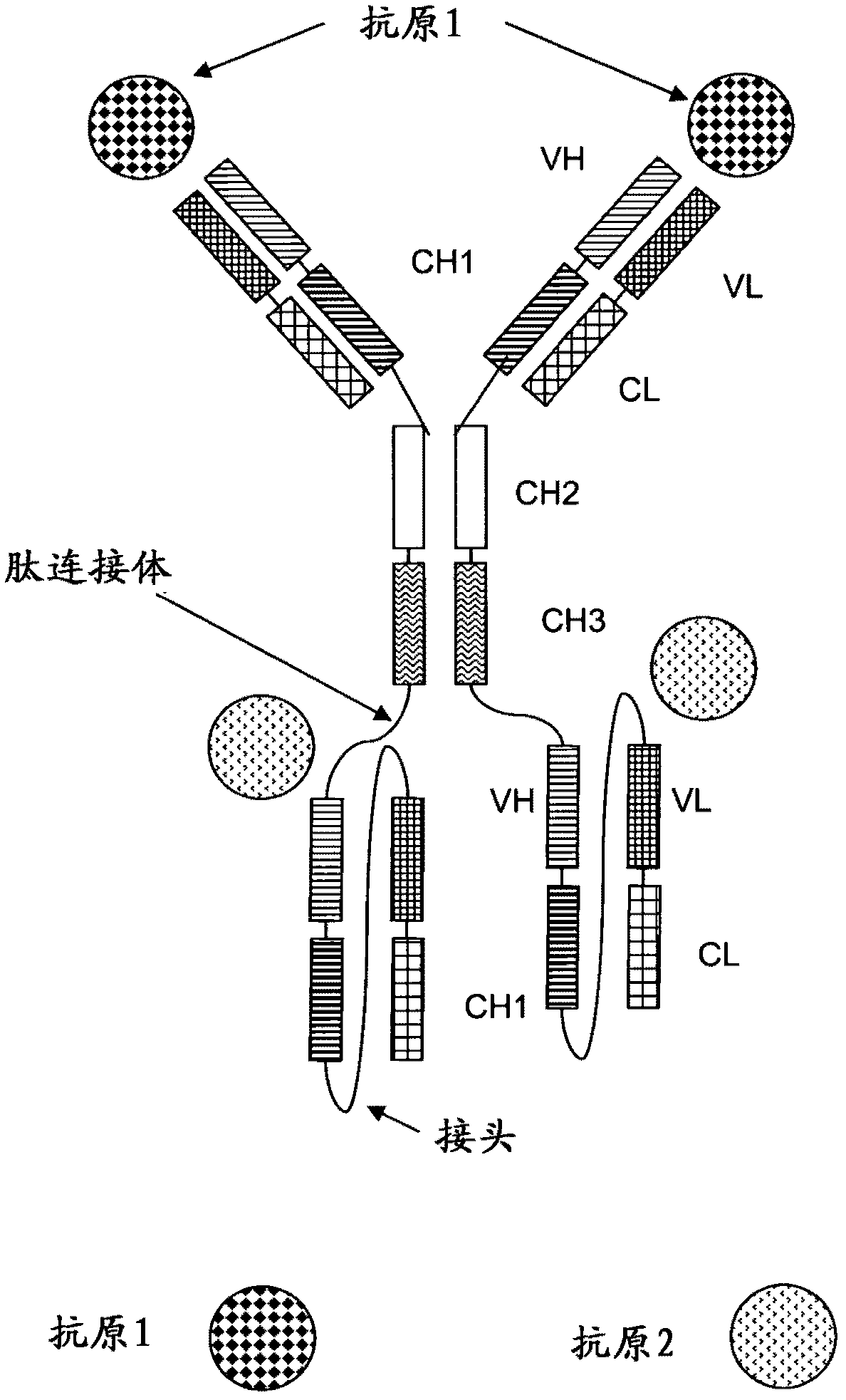
[0249] In the following as one embodiment of the invention, a tetravalent bispecific antibody comprising a full-length antibody binding to a first antigen (IGF-1R or EGFR) and two single-chain Fab fragments, the two Single-chain Fab fragments are linked to full-length antibodies (both single-chain Fab fragments are at both C-terminal ends of the heavy chain or at both C-terminal ends of the light chain) via a peptide linker and linked to a second, different antigen (IGF-1R or Another) binding of EGFR. The antibody domains and linker have the following order in N-terminal to C-terminal direction in the single chain Fab fragment: VL-CL-Linker-VH-CH1.
[0250] as used for For the heavy chain variable region VH of the antigen binding site, SEQ ID NO:15 was used. as used for For the light chain variable region VL of the antigen bind...
Embodiment 2
[0259] bispecific Expression & purification of antibody scFabXGFR1 molecule
[0260] The light and heavy chains of the corresponding bispecific antibodies are constructed in expression vectors carrying prokaryotic and eukaryotic selectable markers. These plasmids were amplified in E. coli, purified and subsequently transfected into HEK293F cells for transient expression of recombinant proteins (using Invitrogen's freesyle system). After 7 days, HEK 293 cell supernatants were obtained and purified by protein A and size exclusion chromatography. The homogeneity of all bispecific antibody constructs was confirmed by SDS-PAGE under non-reducing and reducing conditions. Under reducing conditions ( Figure 8 ), polypeptide chains carrying C- and N-terminal scFab fusions showed surface molecular sizes based on SDS-PAGE similar to the calculated molecular weights. The expression levels of all constructs were analyzed by protein A HPLC and were similar to or in some cases slig...
Embodiment 3
[0269] bispecific Stability and aggregation tendency of antibody scFab-XGFR molecules
[0270] HP size exclusion chromatography was performed to determine the amount of aggregates present in the prepared recombinant antibody derivatives. For this, bispecific antibody samples were analyzed by high performance SEC on an UltiMate 3000 HPLC system (Dionex) using a Superdex 200 analytical size exclusion column (GE Healthcare, Sweden). Figure 9 shows examples of these analyses. Aggregates appear as separate peaks or shoulders preceding the fractions containing monomeric antibody derivatives. For this work, we defined a desired 'monomer' molecule consisting of two heterodimers comprising a heavy chain and a light chain with a scFab attached to either of them. After removal of N-glycans by enzymatic treatment with Peptide-N-Glycosidase F (Roche Molecular Biochemicals), the integrity of the reduced bispecific antibody light and heavy chains and the amino acid backbone of the fus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com