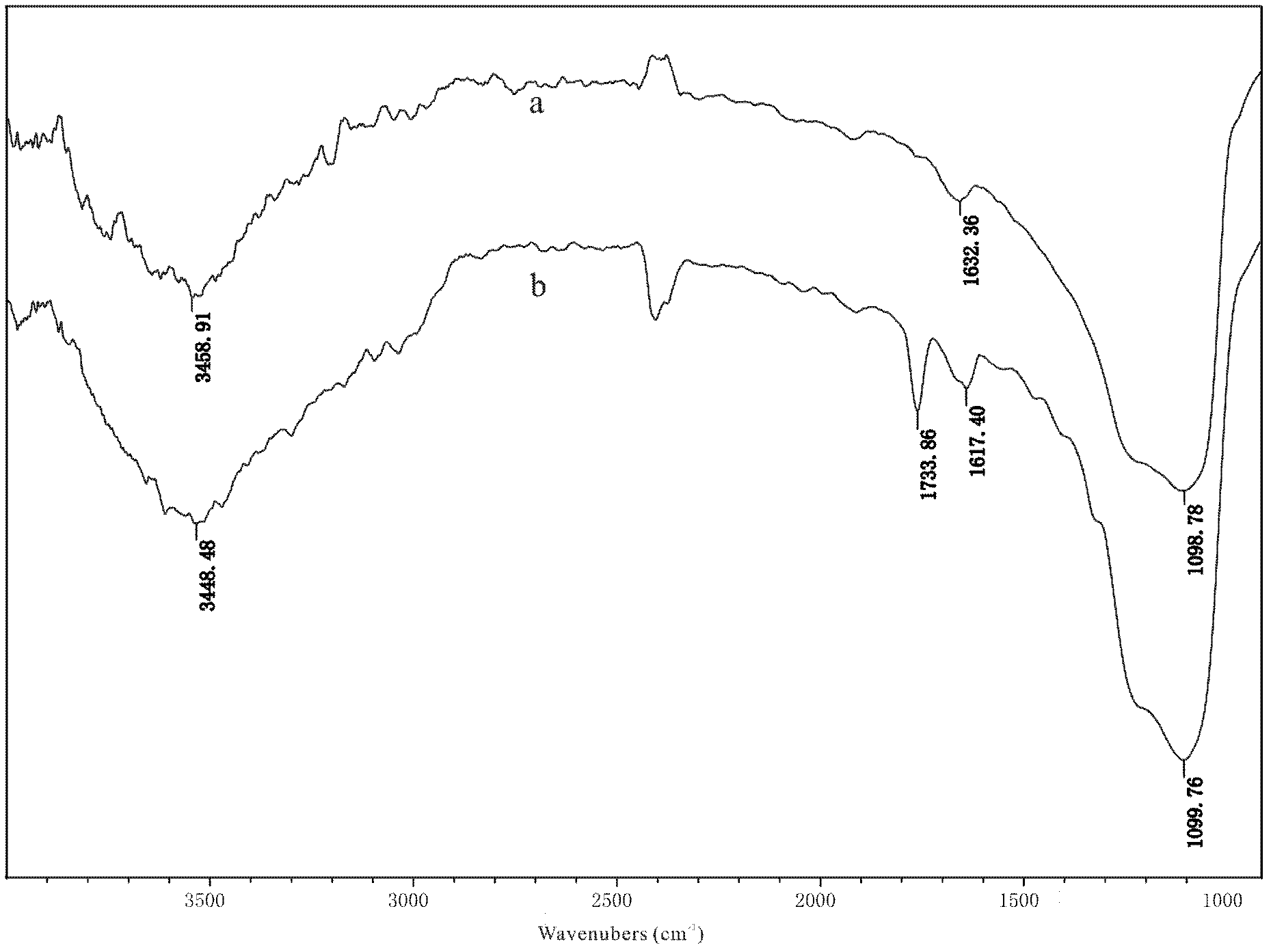
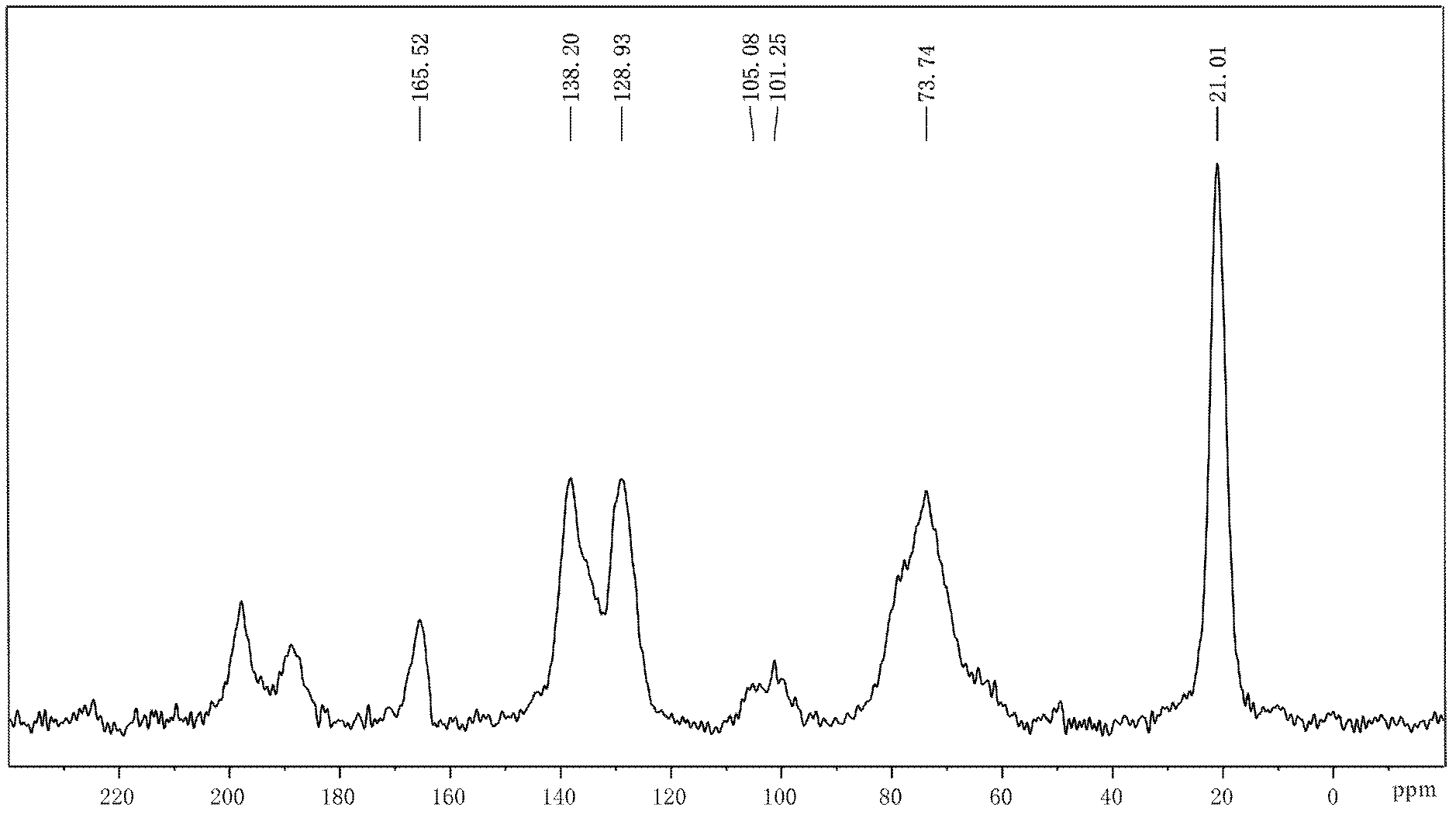
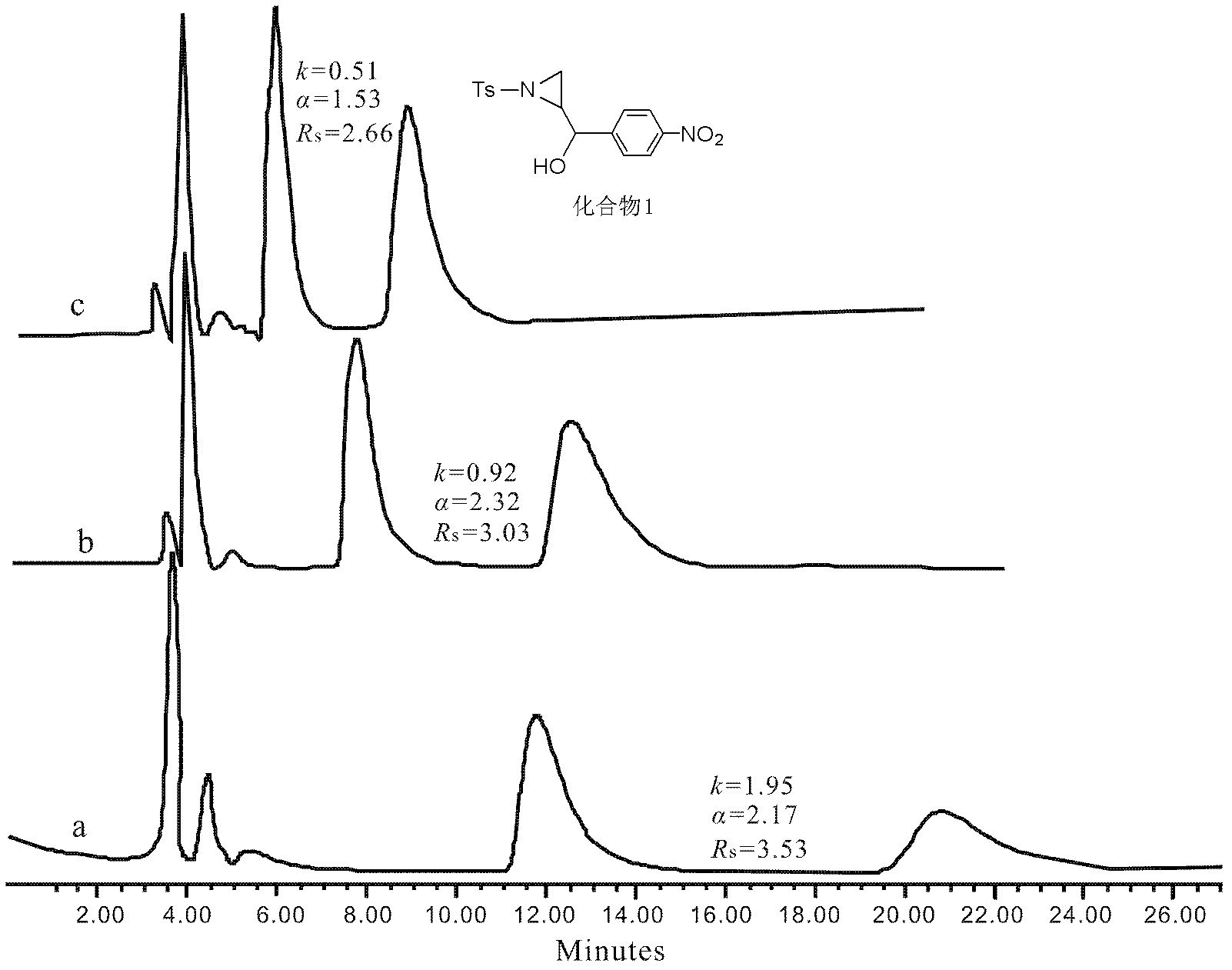
Preparation method of coating-type polysaccharide chiral stationary phase
A technology of chiral stationary phase and polysaccharides, applied in chemical instruments and methods, other chemical processes, etc., can solve the problems of inability to coat polysaccharide derivatives, inability to prepare chiral immobilization, etc., and achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A method for preparing a coated polysaccharide chiral stationary phase (or called: cellulose-tris(3,5-dimethylbenzoate)-silica gel composite chiral stationary phase), specific steps as follows:
[0044] 1) Preparation of cellulose solution,
[0045] First put the dry cellulose (0.70g) in a three-necked flask, add dimethylacetamide (25mL), protect it under nitrogen, stir and activate at 160°C for 1h, cool, filter, and wash with acetone several times, and then the fiber The element was dried under normal pressure at 60°C. Take dry LiCl (6.50g) (dried at 200°C for 4h before use) and dimethylacetamide (65mL) in a three-necked flask, heat to 100°C, after LiCl is completely dissolved in dimethylacetamide, add dry For good cellulose, cool down to 80°C, stir to dissolve for 3 hours, and cool to room temperature. Then the cooled solution was heated to 80°C, stirred for 2h, and cooled to room temperature. The cooled solution was centrifuged to obtain a clear cellulose solutio...
Embodiment 2
[0055] Embodiment 2: Preparation of starch-three (3,5-dimethylphenylcarbamate)-silica gel composite chiral stationary phase,
[0056] 1) preparation of starch solution,
[0057] Amylose (4.02g) was added into cold distilled water (396mL), then heated to boil, stirred to dissolve, and cooled to obtain a 1wt% clear starch solution.
[0058] 2) coating of starch (preparation of starch-silica gel composite),
[0059] The obtained starch solution (380.47g) was divided into 7 equal parts, and the first part was mixed with dry blank silica gel (13.49g), and then distilled under reduced pressure at 70°C with a rotary evaporator. After evaporating to dryness, repeat the above-mentioned coating operation 6 times with the remaining starch solution, and use the same amount of starch solution each time to obtain a white powdery product (starch-silica gel composite 17.21g) after drying, and the coating amount is 28.2%. .
[0060] 3) Preparation of starch-tris(3,5-dimethylphenylcarbamate)...
Embodiment 3
[0067] Preparation of chiral stationary phase of chitosan-tris(4-methylphenylcarbamate)-silica gel composite
[0068] 1) preparation of chitosan solution,
[0069] Weigh 2.52g of chitosan and add it into 1wt% hydrochloric acid (198mL), stir and dissolve to obtain a 1wt% clear chitosan solution.
[0070] 2) coating of chitosan (preparation of chitosan-silica gel composite),
[0071]Get the prepared chitosan solution (180.41g) and dry blank silica gel (10.42g) and mix evenly, under the condition of stirring, dropwise add precipitating agent sodium hydroxide solution (1wt%) until the solution becomes neutral, filter and wash with distilled water 3 times until chloride ions are no longer detected with an aqueous solution of silver nitrate. After drying, a white powdery product (12.04 g of chitosan-silica gel composite) was obtained, and the coating amount was 14.8%.
[0072] 3) Preparation of chitosan-tris(4-methylphenylcarbamate)-silica gel complex (derivatization of chitosan-...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap