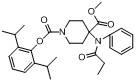
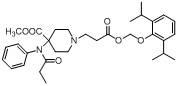
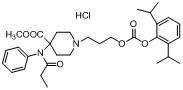
4-(methoxycarbonyl)-4-(N- phenylpropionamido)- piperidine-1-substituted compound, preparation method and pharmaceutical application
A technology of phenylpropanamide and methoxycarbonyl, applied in the field of 4--4--piperidine-1-substituted compounds, which can solve the problem of low water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 2,6-Diisopropylphenol (IV) (9 mmol) was dissolved in THF (20 ml), 0 o C. Under the protection of nitrogen, 6 ml of tetrahydrofuran solution containing 21% potassium hexamethyldisilazide (KHMDS) was added dropwise. Reaction solution at 0 o Stir at C for 40 min, then add dropwise 5 ml of a tetrahydrofuran solution containing terminally brominated acid chloride (18 mmol). The reaction solution was stirred overnight at room temperature, then poured into a large amount of water, extracted with ethyl acetate, separated the organic layer, washed the organic layer once with brine, dried the organic layer with anhydrous sodium sulfate, filtered, evaporated the filtrate to dryness under reduced pressure, and the residue The product was purified by silica gel column chromatography (developing solvent: cyclohexane / ethyl acetate = 40:1) to obtain a yellow oil product intermediate (2,6-diisopropylphenol ω-bromocarboxylate or 2,6 - diisopropylphenol acrylate). Wherein when n=1, the...
Embodiment 2
[0041] Dissolve the compound of formula (Ⅴ) (RP-acid) (100 mg, 0.28 mmol) in 20 ml of acetone, add propofol chloromethyl ether (CAS: 22, 94 mg, 0.42 mmol), Cs 2 CO 3 (180 mg, 0.55 mmol). Reflux overnight after the completion of the reaction, cool the reaction solution to room temperature, filter, concentrate the filtrate, and purify the residue with preparative thin layer (developing solvent: cyclohexane / ethyl acetate = 1.5:1) to obtain the free base in the form of formula (I) Colorless oily product 100mg, yield 65%. The reaction process is as follows:
[0042] .
[0043] Product structure detection results:
[0044] 1 HNMR(δ)(CDCl 3 ): 0.928~0.976 (t, 3H), 1.183~1.206(2s,12H), 1.592~1.630(t,2H), 1.829~1.903 (q, 2H), 2.246~2.290 (d, 2H), 2.384~2.507 (m, 4H), 2.581~2.680 (m,4H), 3.257~3.303(m,2H), 5.526(s,2H), 7.092~7.123(t,3H), 7.263~7.415(m,5H).
[0045] Elemental analysis (instrument: Italian CARLO ERBA 1106 elemental analyzer): N (4.92%), C (69.21%), H (8.06%)...
Embodiment 3
[0047] The reaction process is as follows:
[0048] Under nitrogen protection, dissolve propofol chloroformate (0.6 ml, 0.34 mmol) in toluene, add triethylamine (52 mg, 0.52 mmol) (or pyridine 0.52 mmol), add formula (Ⅵ) compound (60 mg, 0.17 mmol) in dichloromethane solution 10 ml. The reaction was stirred overnight. After concentration, 23 mg of the free base yellow oily product in the form of formula (I) was obtained by thin layer preparation (developing solvent: cyclohexane / ethyl acetate = 1:1), with a yield of 24.5%. The reaction process is as follows:
[0049] .
[0050] Dissolve 23 mg of the free base product of formula (I) in 10 ml of anhydrous ether, and pass through dry hydrogen chloride gas at 0°C for 2 h, concentrate and filter to obtain 21 mg of its hydrochloride product as a white solid, with a yield of 84.9%.
[0051] The hydrochloride product structure detection result:
[0052] 1 HNMR(δ)(CD 3 OD): 0.831~0.868 (t, 3H), 1.078~1.095(2s,12H), 1.822~1...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap